The myosin chaperone UNC-45 is organized in tandem modules to support myofilament formation in C. elegans
- PMID: 23332754
- PMCID: PMC3549490
- DOI: 10.1016/j.cell.2012.12.025
The myosin chaperone UNC-45 is organized in tandem modules to support myofilament formation in C. elegans
Abstract
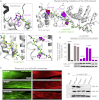
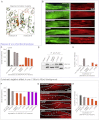
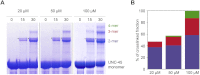
The UCS (UNC-45/CRO1/She4) chaperones play an evolutionarily conserved role in promoting myosin-dependent processes, including cytokinesis, endocytosis, RNA transport, and muscle development. To investigate the protein machinery orchestrating myosin folding and assembly, we performed a comprehensive analysis of Caenorhabditis elegans UNC-45. Our structural and biochemical data demonstrate that UNC-45 forms linear protein chains that offer multiple binding sites for cooperating chaperones and client proteins. Accordingly, Hsp70 and Hsp90, which bind to the TPR domain of UNC-45, could act in concert and with defined periodicity on captured myosin molecules. In vivo analyses reveal the elongated canyon of the UCS domain as a myosin-binding site and show that multimeric UNC-45 chains support organization of sarcomeric repeats. In fact, expression of transgenes blocking UNC-45 chain formation induces dominant-negative defects in the sarcomere structure and function of wild-type worms. Together, these findings uncover a filament assembly factor that directly couples myosin folding with myofilament formation.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures











References
-
- Barral J.M., Hutagalung A.H., Brinker A., Hartl F.U., Epstein H.F. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–671. - PubMed
-
- Craig R., Woodhead J.L. Structure and function of myosin filaments. Curr. Opin. Struct. Biol. 2006;16:204–212. - PubMed
Supplemental References
-
- Abrahams, J.P., and Leslie, A.G. (1996). Methods used in the structure determination of bovine mitochondrial F1 ATPase. Acta Crystallogr. D Biol. Crystallogr. 52, 30–42. - PubMed
-
- Abramoff, M.D., Magalhães, P.J., and Ram, S.J. (2004). Image processing with ImageJ. Biophotonics Intl. 11, 36–42.
-
- Adams, P.D., Grosse-Kunstleve, R.W., Hung, L.W., Ioerger, T.R., McCoy, A.J., Moriarty, N.W., Read, R.J., Sacchettini, J.C., Sauter, N.K., and Terwilliger, T.C. (2002). PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

