Volume-conserving trans-cis isomerization pathways in photoactive yellow protein visualized by picosecond X-ray crystallography
- PMID: 23422563
- PMCID: PMC3579544
- DOI: 10.1038/nchem.1565
Volume-conserving trans-cis isomerization pathways in photoactive yellow protein visualized by picosecond X-ray crystallography
Abstract
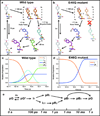
Trans-to-cis isomerization, the key reaction in photoactive proteins, usually cannot occur through the standard one-bond-flip mechanism. Owing to spatial constraints imposed by a protein environment, isomerization probably proceeds through a volume-conserving mechanism in which highly choreographed atomic motions are expected, the details of which have not yet been observed directly. Here we employ time-resolved X-ray crystallography to visualize structurally the isomerization of the p-coumaric acid chromophore in photoactive yellow protein with a time resolution of 100 ps and a spatial resolution of 1.6 Å. The structure of the earliest intermediate (I(T)) resembles a highly strained transition state in which the torsion angle is located halfway between the trans- and cis-isomers. The reaction trajectory of I(T) bifurcates into two structurally distinct cis intermediates via hula-twist and bicycle-pedal pathways. The bifurcating reaction pathways can be controlled by weakening the hydrogen bond between the chromophore and an adjacent residue through E46Q mutation, which switches off the bicycle-pedal pathway.
Figures
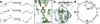



Comment in
-
Contradictions in X-ray structures of intermediates in the photocycle of photoactive yellow protein.Nat Chem. 2014 Apr;6(4):258-9. doi: 10.1038/nchem.1898. Nat Chem. 2014. PMID: 24651178 Free PMC article. No abstract available.
-
Reply to 'contradictions in X-ray structures of intermediates in the photocycle of photoactive yellow protein'.Nat Chem. 2014 Apr;6(4):259-60. doi: 10.1038/nchem.1897. Nat Chem. 2014. PMID: 24651179 Free PMC article. No abstract available.
References
-
- Warshel A, Barboy N. Energy storage and reaction pathways in the first step of the vision process. J. Am. Chem. Soc. 1982;104:1469–1476.
-
- Muller AM, Lochbrunner S, Schmid WE, Fuss W. Low-temperature photochemistry of previtamin D: A hula-twist isomerization of a triene. Angew. Chem. Int. Ed. 1998;37:505–507. - PubMed
-
- Liu RS, Yang LY, Liu J. Mechanisms of photoisomerization of polyenes in confined media: from organic glasses to protein binding cavities. Photochem. Photobiol. 2007;83:2–10. - PubMed
-
- Imamoto Y, Kataoka M, Liu RS. Mechanistic pathways for the photoisomerization reaction of the anchored, tethered chromophore of the photoactive yellow protein and its mutants. Photochem. Photobiol. 2002;76:584–589. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

