Structural basis for carbapenemase activity of the OXA-23 β-lactamase from Acinetobacter baumannii
- PMID: 24012371
- PMCID: PMC3888872
- DOI: 10.1016/j.chembiol.2013.07.015
Structural basis for carbapenemase activity of the OXA-23 β-lactamase from Acinetobacter baumannii
Abstract
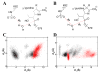
Dissemination of Acinetobacter baumannii strains harboring class D β-lactamases producing resistance to carbapenem antibiotics severely limits our ability to treat deadly Acinetobacter infections. Susceptibility determination in the A. baumannii background and kinetic studies with a homogeneous preparation of OXA-23 β-lactamase, the major carbapenemase present in A. baumannii, document the ability of this enzyme to manifest resistance to last-resort carbapenem antibiotics. We also report three X-ray structures of OXA-23: apo OXA-23 at two different pH values, and wild-type OXA-23 in complex with meropenem, a carbapenem substrate. The structures and dynamics simulations reveal an important role for Leu166, whose motion regulates the access of a hydrolytic water molecule to the acyl-enzyme species in imparting carbapenemase activity.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
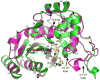
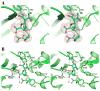
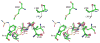

References
-
- Abbo A, Carmeli Y, Navon-Venezia S, Siegman-Igra Y, Schwaber MJ. Impact of multi-drug-resistant Acinetobacter baumannii on clinical outcomes. Eur J Clin Microbiol Infect Dis. 2007;26:793–800. - PubMed
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. 2010;D66:213–221. - PMC - PubMed
-
- Bayly CI, Cieplak P, Cornell WD, Kollman PA. A well-behaved electrostatic potential based method using charge restraints for determining atom-centered charges: The RESP model. J Phys Chem. 1993;97:10269–10280.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

