Structural basis for biofilm formation via the Vibrio cholerae matrix protein RbmA
- PMID: 23687270
- PMCID: PMC3697633
- DOI: 10.1128/JB.00374-13
Structural basis for biofilm formation via the Vibrio cholerae matrix protein RbmA
Abstract
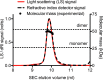
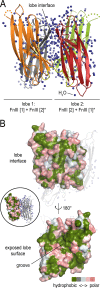
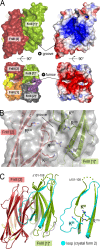
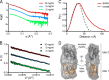
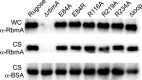
During the transition from a free-swimming, single-cell lifestyle to a sessile, multicellular state called a biofilm, bacteria produce and secrete an extracellular matrix comprised of nucleic acids, exopolysaccharides, and adhesion proteins. The Vibrio cholerae biofilm matrix contains three major protein components, RbmA, Bap1, and RbmC, which are unique to Vibrio cholerae and appear to support biofilm formation at particular steps in the process. Here, we focus on RbmA, a structural protein with an unknown fold. RbmA participates in the early cell-cell adhesion events and is found throughout the biofilm where it localizes to cell-cell contact sites. We determined crystal structures of RbmA and revealed that the protein folds into tandem fibronectin type III (FnIII) folds. The protein is dimeric in solution and in crystals, with the dimer interface displaying a surface groove that is lined with several positively charged residues. Structure-guided mutagenesis studies establish a crucial role for this surface patch for RbmA function. On the basis of the structure, we hypothesize that RbmA serves as a tether by maintaining flexible linkages between cells and the extracellular matrix.
Figures
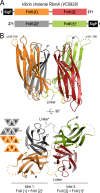



References
-
- Parsek MR, Singh PK. 2003. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 57:677–701 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

