Probing the Solution Structure of IκB Kinase (IKK) Subunit γ and Its Interaction with Kaposi Sarcoma-associated Herpes Virus Flice-interacting Protein and IKK Subunit β by EPR Spectroscopy
- PMID: 25979343
- PMCID: PMC4505408
- DOI: 10.1074/jbc.M114.622928
Probing the Solution Structure of IκB Kinase (IKK) Subunit γ and Its Interaction with Kaposi Sarcoma-associated Herpes Virus Flice-interacting Protein and IKK Subunit β by EPR Spectroscopy
Erratum in
-
Probing the solution structure of IκB kinase (IKK) subunit γ and its interaction with Kaposi sarcoma-associated herpes virus Flice-interacting protein and IKK subunit β by EPR spectroscopy.J Biol Chem. 2015 Sep 18;290(38):23024. doi: 10.1074/jbc.A114.622928. J Biol Chem. 2015. PMID: 26386046 Free PMC article. No abstract available.
Abstract
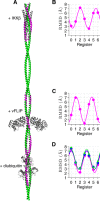
Viral flice-interacting protein (vFLIP), encoded by the oncogenic Kaposi sarcoma-associated herpes virus (KSHV), constitutively activates the canonical nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) pathway. This is achieved through subversion of the IκB kinase (IKK) complex (or signalosome), which involves a physical interaction between vFLIP and the modulatory subunit IKKγ. Although this interaction has been examined both in vivo and in vitro, the mechanism by which vFLIP activates the kinase remains to be determined. Because IKKγ functions as a scaffold, recruiting both vFLIP and the IKKα/β subunits, it has been proposed that binding of vFLIP could trigger a structural rearrangement in IKKγ conducive to activation. To investigate this hypothesis we engineered a series of mutants along the length of the IKKγ molecule that could be individually modified with nitroxide spin labels. Subsequent distance measurements using electron paramagnetic resonance spectroscopy combined with molecular modeling and molecular dynamics simulations revealed that IKKγ is a parallel coiled-coil whose response to binding of vFLIP or IKKβ is localized twisting/stiffening and not large-scale rearrangements. The coiled-coil comprises N- and C-terminal regions with distinct registers accommodated by a twist: this structural motif is exploited by vFLIP, allowing it to bind and subsequently activate the NF-κB pathway. In vivo assays confirm that NF-κB activation by vFLIP only requires the N-terminal region up to the transition between the registers, which is located directly C-terminal of the vFLIP binding site.
Keywords: IKKγ; NEMO; NF-kappa B (NF-κB); electron paramagnetic resonance (EPR); molecular dynamics; molecular modeling; protein structure; signalosome; vFLIP; virology.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Scheidereit C. (2006) IκB kinase complexes: gateways to NF-κB activation and transcription. Oncogene 25, 6685–6705 - PubMed
-
- DiDonato J. A., Hayakawa M., Rothwarf D. M., Zandi E., Karin M. (1997) A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388, 548–554 - PubMed
-
- Mercurio F., Zhu H., Murray B. W., Shevchenko A., Bennett B. L., Li J., Young D. B., Barbosa M., Mann M., Manning A., Rao A. (1997) IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278, 860–866 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

