Integration of Fourier Transform Infrared Spectroscopy, Fluorescence Spectroscopy, Steady-state Kinetics and Molecular Dynamics Simulations of Gαi1 Distinguishes between the GTP Hydrolysis and GDP Release Mechanism
- PMID: 25979337
- PMCID: PMC4498047
- DOI: 10.1074/jbc.M115.651190
Integration of Fourier Transform Infrared Spectroscopy, Fluorescence Spectroscopy, Steady-state Kinetics and Molecular Dynamics Simulations of Gαi1 Distinguishes between the GTP Hydrolysis and GDP Release Mechanism
Abstract
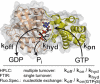
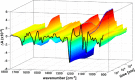
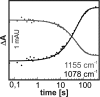
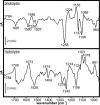
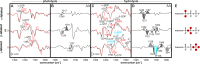
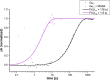
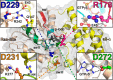
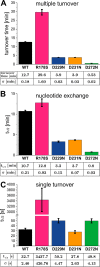
Gα subunits are central molecular switches in cells. They are activated by G protein-coupled receptors that exchange GDP for GTP, similar to small GTPase activation mechanisms. Gα subunits are turned off by GTP hydrolysis. For the first time we employed time-resolved FTIR difference spectroscopy to investigate the molecular reaction mechanisms of Gαi1. FTIR spectroscopy is a powerful tool that monitors reactions label free with high spatio-temporal resolution. In contrast to common multiple turnover assays, FTIR spectroscopy depicts the single turnover GTPase reaction without nucleotide exchange/Mg(2+) binding bias. Global fit analysis resulted in one apparent rate constant of 0.02 s(-1) at 15 °C. Isotopic labeling was applied to assign the individual phosphate vibrations for α-, β-, and γ-GTP (1243, 1224, and 1156 cm(-1), respectively), α- and β-GDP (1214 and 1134/1103 cm(-1), respectively), and free phosphate (1078/991 cm(-1)). In contrast to Ras · GAP catalysis, the bond breakage of the β-γ-phosphate but not the Pi release is rate-limiting in the GTPase reaction. Complementary common GTPase assays were used. Reversed phase HPLC provided multiple turnover rates and tryptophan fluorescence provided nucleotide exchange rates. Experiments were complemented by molecular dynamics simulations. This broad approach provided detailed insights at atomic resolution and allows now to identify key residues of Gαi1 in GTP hydrolysis and nucleotide exchange. Mutants of the intrinsic arginine finger (Gαi1-R178S) affected exclusively the hydrolysis reaction. The effect of nucleotide binding (Gαi1-D272N) and Ras-like/all-α interface coordination (Gαi1-D229N/Gαi1-D231N) on the nucleotide exchange reaction was furthermore elucidated.
Keywords: Fourier transform IR (FTIR); GTPase; enzyme mechanism; fluorescence; heterotrimeric G protein; molecular dynamics; mutagenesis; nucleotide exchange.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Sato M., Blumer J. B., Simon V., Lanier S. M. (2006) Accessory proteins for G proteins: partners in signaling. Annu. Rev. Pharmacol. Toxicol. 46, 151–187 - PubMed
-
- Tall G. G., Krumins A. M., Gilman A. G. (2003) Mammalian Ric-8A (synembryn) is a heterotrimeric Gα protein guanine nucleotide exchange factor. J. Biol. Chem. 278, 8356–8362 - PubMed
-
- Coleman D. E., Berghuis A. M., Lee E., Linder M. E., Gilman A. G., Sprang S. R. (1994) Structures of active conformations of Giα1 and the mechanism of GTP hydrolysis. Science 265, 1405–1412 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

