Structure of the apo form of Bacillus stearothermophilus phosphofructokinase
- PMID: 22212099
- PMCID: PMC3285294
- DOI: 10.1021/bi201548p
Structure of the apo form of Bacillus stearothermophilus phosphofructokinase
Abstract
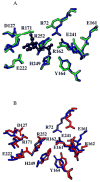
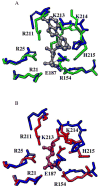
The crystal structure of the unliganded form of Bacillus stearothermophilus phosphofructokinase (BsPFK) was determined using molecular replacement to 2.8 Å resolution (Protein Data Bank entry 3U39 ). The apo BsPFK structure serves as the basis for the interpretation of any structural changes seen in the binary or ternary complexes. When the apo BsPFK structure is compared with the previously published liganded structures of BsPFK, the structural impact that the binding of the ligands produces is revealed. This comparison shows that the apo form of BsPFK resembles the substrate-bound form of BsPFK, a finding that differs from previous predictions.
Figures
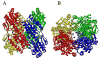

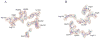
References
-
- Krauss G. Biochemistry of signal transduction and regulation. 2. Wiley-VCH; Weinheim; New York: 2001.
-
- Voet D, Voet JG, Pratt CW. Fundamentals of biochemistry: life at the molecular level. 3. John Wiley & Sons; Hoboken, N.J: 2008.
-
- Kemp RG, Foe LG. Allosteric regulatory properties of muscle phosphofructokinase. Mol Cell Biochem. 1983;57:147–154. - PubMed
-
- Byrnes M, Zhu X, Younathan ES, Chang SH. Kinetic characteristics of phosphofructokinase from Bacillus stearothermophilus: MgATP nonallosterically inhibits the enzyme. Biochemistry. 1994;33:3424–3431. - PubMed
-
- Blangy D, Buc H, Monod J. Kinetics of the allosteric interactions of phosphofructokinase from Escherichia coli. J Mol Biol. 1968;31:13–35. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

