Structural basis for cooperative interactions of substituted 2-aminopyrimidines with the acetylcholine binding protein
- PMID: 25006260
- PMCID: PMC4115551
- DOI: 10.1073/pnas.1410992111
Structural basis for cooperative interactions of substituted 2-aminopyrimidines with the acetylcholine binding protein
Abstract
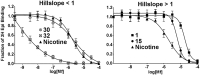
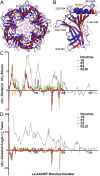
The nicotinic acetylcholine receptor (nAChR) and the acetylcholine binding protein (AChBP) are pentameric oligomers in which binding sites for nicotinic agonists and competitive antagonists are found at selected subunit interfaces. The nAChR spontaneously exists in multiple conformations associated with its activation and desensitization steps, and conformations are selectively stabilized by binding of agonists and antagonists. In the nAChR, agonist binding and the associated conformational changes accompanying activation and desensitization are cooperative. AChBP, which lacks the transmembrane spanning and cytoplasmic domains, serves as a homology model of the extracellular domain of the nAChRs. We identified unique cooperative binding behavior of a number of 4,6-disubstituted 2-aminopyrimidines to Lymnaea AChBP, with different molecular variants exhibiting positive, nH > 1.0, and negative cooperativity, nH < 1.0. Therefore, for a distinctive set of ligands, the extracellular domain of a nAChR surrogate suffices to accommodate cooperative interactions. X-ray crystal structures of AChBP complexes with examples of each allowed the identification of structural features in the ligands that confer differences in cooperative behavior. Both sets of molecules bind at the agonist-antagonist site, as expected from their competition with epibatidine. An analysis of AChBP quaternary structure shows that cooperative ligand binding is associated with a blooming or flare conformation, a structural change not observed with the classical, noncooperative, nicotinic ligands. Positively and negatively cooperative ligands exhibited unique features in the detailed binding determinants and poses of the complexes.
Keywords: allostery; crystallography; nicotinic receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

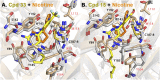
References
-
- Changeux JP. 50 years of allosteric interactions: The twists and turns of the models. Nat Rev Mol Cell Biol. 2013;14(12):819–829. - PubMed
-
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3(2):102–114. - PubMed
-
- Thompson AJ, Lester HA, Lummis SC. The structural basis of function in Cys-loop receptors. Q Rev Biophys. 2010;43(4):449–499. - PubMed
-
- Corringer PJ, et al. Structure and pharmacology of pentameric receptor channels: From bacteria to brain. Structure. 2012;20(6):941–956. - PubMed
-
- Heidmann T, Changeux JP. Fast kinetic studies on the interaction of a fluorescent agonist with the membrane-bound acetylcholine receptor from Torpedo marmorata. Eur J Biochem. 1979;94(1):255–279. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

