Structure, biochemistry, and inhibition of essential 4'-phosphopantetheinyl transferases from two species of Mycobacteria
- PMID: 24963544
- PMCID: PMC4168790
- DOI: 10.1021/cb500263p
Structure, biochemistry, and inhibition of essential 4'-phosphopantetheinyl transferases from two species of Mycobacteria
Abstract
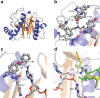
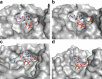
4'-Phosphopantetheinyl transferases (PPTase) post-translationally modify carrier proteins with a phosphopantetheine moiety, an essential reaction in all three domains of life. In the bacterial genus Mycobacteria, the Sfp-type PPTase activates pathways necessary for the biosynthesis of cell wall components and small molecule virulence factors. We solved the X-ray crystal structures and biochemically characterized the Sfp-type PPTases from two of the most prevalent Mycobacterial pathogens, PptT of M. tuberculosis and MuPPT of M. ulcerans. Structural analyses reveal significant differences in cofactor binding and active site composition when compared to previously characterized Sfp-type PPTases. Functional analyses including the efficacy of Sfp-type PPTase-specific inhibitors also suggest that the Mycobacterial Sfp-type PPTases can serve as therapeutic targets against Mycobacterial infections.
Figures

References
-
- Wilson M. L. (2008) Reducing the global burden of mycobacterial infections: one more piece of the puzzle. Am. J. Clin. Pathol. 130, 849–852. - PubMed
-
- Almeida Da Silva P. E.; Palomino J. C. (2011) Molecular basis and mechanisms of drug resistance in Mycobacterium tuberculosis: classical and new drugs. J. Antimicrob. Chemother. 66, 1417–1430. - PubMed
-
- Wansbrough-Jones M.; Phillips R. (2006) Buruli ulcer: emerging from obscurity. Lancet 367, 1849–1858. - PubMed
-
- Brennan P. J. (2003) Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberculosis 83, 91–97. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

