How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation
- PMID: 25190797
- PMCID: PMC4242719
- DOI: 10.1126/science.1255030
How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation
Abstract
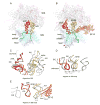
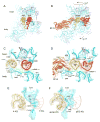
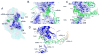
Coupled translocation of messenger RNA and transfer RNA (tRNA) through the ribosome, a process catalyzed by elongation factor EF-G, is a crucial step in protein synthesis. The crystal structure of a bacterial translocation complex describes the binding states of two tRNAs trapped in mid-translocation. The deacylated P-site tRNA has moved into a partly translocated pe/E chimeric hybrid state. The anticodon stem-loop of the A-site tRNA is captured in transition toward the 30S P site, while its 3' acceptor end contacts both the A and P loops of the 50S subunit, forming an ap/ap chimeric hybrid state. The structure shows how features of ribosomal RNA rearrange to hand off the A-site tRNA to the P site, revealing an active role for ribosomal RNA in the translocation process.
Copyright © 2014, American Association for the Advancement of Science.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

