Crystal structures of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design
- PMID: 25492868
- PMCID: PMC4318995
- DOI: 10.1074/jbc.M114.624536
Crystal structures of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design
Abstract
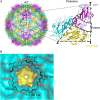
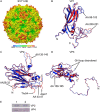
Hand-foot-and-mouth disease (HFMD) remains a major health concern in the Asia-Pacific regions, and its major causative agents include human enterovirus 71 (EV71) and coxsackievirus A16. A desirable vaccine against HFMD would be multivalent and able to elicit protective responses against multiple HFMD causative agents. Previously, we have demonstrated that a thermostable recombinant EV71 vaccine candidate can be produced by the insertion of a foreign peptide into the BC loop of VP1 without affecting viral replication. Here we present crystal structures of two different naturally occurring empty particles, one from a clinical C4 strain EV71 and the other from its recombinant virus containing an insertion in the VP1 BC loop. Crystal structure analysis demonstrated that the inserted foreign peptide is well exposed on the particle surface without significant structural changes in the capsid. Importantly, such insertions do not seem to affect the virus uncoating process as illustrated by the conformational similarity between an uncoating intermediate of another recombinant virus and that of EV71. Especially, at least 18 residues from the N terminus of VP1 are transiently externalized. Altogether, our study provides insights into vaccine development against HFMD.
Keywords: Crystal Structure; Infectious Disease; Vaccine; Vaccine Development; Virus Structure.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- McMinn P. C. (2012) Recent advances in the molecular epidemiology and control of human enterovirus 71 infection. Curr. Opin. Virol. 2, 199–205 - PubMed
-
- Wang G., Cao R. Y., Chen R., Mo L., Han J. F., Wang X., Xu X., Jiang T., Deng Y. Q., Lyu K., Zhu S. Y., Qin E. D., Tang R., Qin C. F. (2013) Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc. Natl. Acad. Sci. U.S.A. 110, 7619–7624 - PMC - PubMed
-
- Evans D. J., McKeating J., Meredith J. M., Burke K. L., Katrak K., John A., Ferguson M., Minor P. D., Weiss R. A., Almond J. W. (1989) An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature 339, 385–388, 340 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

