Structural and Biochemical Characterization of the Early and Late Enzymes in the Lignin β-Aryl Ether Cleavage Pathway from Sphingobium sp. SYK-6
- PMID: 26940872
- PMCID: PMC4858972
- DOI: 10.1074/jbc.M115.700427
Structural and Biochemical Characterization of the Early and Late Enzymes in the Lignin β-Aryl Ether Cleavage Pathway from Sphingobium sp. SYK-6
Abstract
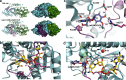
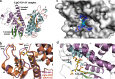
There has been great progress in the development of technology for the conversion of lignocellulosic biomass to sugars and subsequent fermentation to fuels. However, plant lignin remains an untapped source of materials for production of fuels or high value chemicals. Biological cleavage of lignin has been well characterized in fungi, in which enzymes that create free radical intermediates are used to degrade this material. In contrast, a catabolic pathway for the stereospecific cleavage of β-aryl ether units that are found in lignin has been identified in Sphingobium sp. SYK-6 bacteria. β-Aryl ether units are typically abundant in lignin, corresponding to 50-70% of all of the intermonomer linkages. Consequently, a comprehensive understanding of enzymatic β-aryl ether (β-ether) cleavage is important for future efforts to biologically process lignin and its breakdown products. The crystal structures and biochemical characterization of the NAD-dependent dehydrogenases (LigD, LigO, and LigL) and the glutathione-dependent lyase LigG provide new insights into the early and late enzymes in the β-ether degradation pathway. We present detailed information on the cofactor and substrate binding sites and on the catalytic mechanisms of these enzymes, comparing them with other known members of their respective families. Information on the Lig enzymes provides new insight into their catalysis mechanisms and can inform future strategies for using aromatic oligomers derived from plant lignin as a source of valuable aromatic compounds for biofuels and other bioproducts.
Keywords: biodegradation; biofuel; crystal structure; enzyme kinetics; lignin degradation.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Sims R. E. H., Mabee W., Saddler J. N., and Taylor M. (2010) An overview of second generation biofuel technologies. Bioresour. Technol. 101, 1570–1580 - PubMed
-
- Bugg T. D. H., Ahmad M., Hardiman E. M., and Rahmanpour R. (2011) Pathways for degradation of lignin in bacteria and fungi. Nat. Prod. Rep. 28, 1883–1896 - PubMed
-
- Bugg T. D. H., Ahmad M., Hardiman E. M., and Singh R. (2011) The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 22, 394–400 - PubMed
-
- Masai E., Katayama Y., and Fukuda M. (2007) Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci. Biotechnol. Biochem. 71, 1–15 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

