Structural insight into the recognition of pathogen-derived phosphoglycolipids by C-type lectin receptor DCAR
- PMID: 32139512
- PMCID: PMC7186165
- DOI: 10.1074/jbc.RA120.012491
Structural insight into the recognition of pathogen-derived phosphoglycolipids by C-type lectin receptor DCAR
Abstract
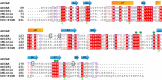
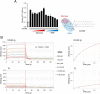
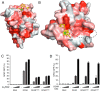
The C-type lectin receptors (CLRs) form a family of pattern recognition receptors that recognize numerous pathogens, such as bacteria and fungi, and trigger innate immune responses. The extracellular carbohydrate-recognition domain (CRD) of CLRs forms a globular structure that can coordinate a Ca2+ ion, allowing receptor interactions with sugar-containing ligands. Although well-conserved, the CRD fold can also display differences that directly affect the specificity of the receptors for their ligands. Here, we report crystal structures at 1.8-2.3 Å resolutions of the CRD of murine dendritic cell-immunoactivating receptor (DCAR, or Clec4b1), the CLR that binds phosphoglycolipids such as acylated phosphatidyl-myo-inositol mannosides (AcPIMs) of mycobacteria. Using mutagenesis analysis, we identified critical residues, Ala136 and Gln198, on the surface surrounding the ligand-binding site of DCAR, as well as an atypical Ca2+-binding motif (Glu-Pro-Ser/EPS168-170). By chemically synthesizing a water-soluble ligand analog, inositol-monophosphate dimannose (IPM2), we confirmed the direct interaction of DCAR with the polar moiety of AcPIMs by biolayer interferometry and co-crystallization approaches. We also observed a hydrophobic groove extending from the ligand-binding site that is in a suitable position to interact with the lipid portion of whole AcPIMs. These results suggest that the hydroxyl group-binding ability and hydrophobic groove of DCAR mediate its specific binding to pathogen-derived phosphoglycolipids such as mycobacterial AcPIMs.
Keywords: C-type lectin domain family 4 member; C-type lectins; Mycobacterium tuberculosis; X-ray crystallography; acylated phosphatidyl-myo-inositol mannoside (AcPIM); glycolipid; innate immunity; myeloid cell; pattern recognition receptor (PRR).
© 2020 Omahdi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


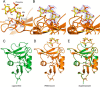
References
-
- Nagata M., Izumi Y., Ishikawa E., Kiyotake R., Doi R., Iwai S., Omahdi Z., Yamaji T., Miyamoto T., Bamba T., and Yamasaki S. (2017) Intracellular metabolite β-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity. Proc. Natl. Acad. Sci. U.S.A. 114, E3285–E3294 10.1073/pnas.1618133114 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

