Crystallographic Snapshots of Class A β-Lactamase Catalysis Reveal Structural Changes That Facilitate β-Lactam Hydrolysis
- PMID: 28100776
- PMCID: PMC5354505
- DOI: 10.1074/jbc.M116.764340
Crystallographic Snapshots of Class A β-Lactamase Catalysis Reveal Structural Changes That Facilitate β-Lactam Hydrolysis
Abstract
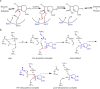
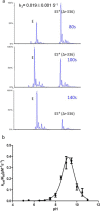
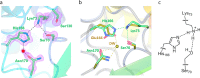
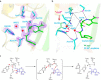
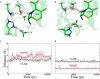
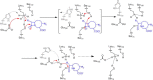
β-Lactamases confer resistance to β-lactam-based antibiotics. There is great interest in understanding their mechanisms to enable the development of β-lactamase-specific inhibitors. The mechanism of class A β-lactamases has been studied extensively, revealing Lys-73 and Glu-166 as general bases that assist the catalytic residue Ser-70. However, the specific roles of these two residues within the catalytic cycle remain not fully understood. To help resolve this, we first identified an E166H mutant that is functional but is kinetically slow. We then carried out time-resolved crystallographic study of a full cycle of the catalytic reaction. We obtained structures that represent apo, ES*-acylation, and ES*-deacylation states and analyzed the conformational changes of His-166. The "in" conformation in the apo structure allows His-166 to form a hydrogen bond with Lys-73. The unexpected "flipped-out" conformation of His-166 in the ES*-acylation structure was further examined by molecular dynamics simulations, which suggested deprotonated Lys-73 serving as the general base for acylation. The "revert-in" conformation in the ES*-deacylation structure aligns His-166 toward the water molecule that hydrolyzes the acyl adduct. Finally, when the acyl adduct is fully hydrolyzed, His-166 rotates back to the "in" conformation of the apo-state, restoring the Lys-73/His-166 interaction. Using His-166 as surrogate, our study identifies distinct conformational changes within the active site during catalysis. We suggest that the native Glu-166 executes similar changes in a less constricted way. Taken together, this structural series improves our understanding of β-lactam hydrolysis in this important class of enzymes.
Keywords: X-ray crystallography; antibiotic resistance; enzyme catalysis; enzyme kinetics; structure-function.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Molton J. S., Tambyah P. A., Ang B. S., Ling M. L., and Fisher D. A. (2013) The global spread of healthcare-associated multidrug-resistant bacteria: a perspective from Asia. Clin. Infect. Dis. 56, 1310–1318 - PubMed
-
- Majiduddin F. K., Materon I. C., and Palzkill T. G. (2002) Molecular analysis of β-lactamase structure and function. Int. J. Med. Microbiol. 292, 127–137 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

