Ion-pulling simulations provide insights into the mechanisms of channel opening of the skeletal muscle ryanodine receptor
- PMID: 28584051
- PMCID: PMC5546034
- DOI: 10.1074/jbc.M116.760199
Ion-pulling simulations provide insights into the mechanisms of channel opening of the skeletal muscle ryanodine receptor
Abstract
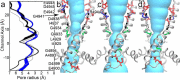
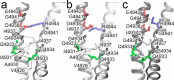
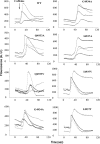
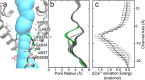
The type 1 ryanodine receptor (RyR1) mediates Ca2+ release from the sarcoplasmic reticulum to initiate skeletal muscle contraction and is associated with muscle diseases, malignant hyperthermia, and central core disease. To better understand RyR1 channel function, we investigated the molecular mechanisms of channel gating and ion permeation. An adequate model of channel gating requires accurate, high-resolution models of both open and closed states of the channel. To this end, we generated an open-channel RyR1 model using molecular simulations to pull Ca2+ through the pore constriction site of a closed-channel RyR1 structure determined at 3.8-Å resolution. Importantly, we find that our open-channel model is consistent with the RyR1 and cardiac RyR (RyR2) open-channel structures reported while this paper was in preparation. Both our model and the published structures show similar rotation of the upper portion of the pore-lining S6 helix away from the 4-fold channel axis and twisting of Ile-4937 at the channel constriction site out of the channel pore. These motions result in a minimum open-channel pore radius of ∼3 Å formed by Gln-4933, rather than Ile-4937 in the closed-channel structure. We also present functional support for our model by mutations around the closed- and open-channel constriction sites (Gln-4933 and Ile-4937). Our results indicate that use of ion-pulling simulations produces a RyR1 open-channel model, which can provide insights into the mechanisms of channel opening complementing those from the structural data.
Keywords: calcium channel; calcium transport; conformational change; molecular dynamics; molecular modeling; ryanodine receptor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
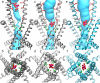
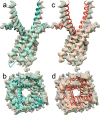
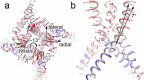

References
-
- Franzini-Armstrong C., and Protasi F. (1997) Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol. Rev. 77, 699–729 - PubMed
-
- McCarthy T. V., Quane K. A., and Lynch P. J. (2000) Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum. Mutat. 15, 410–417 - PubMed
-
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., and Meissner G. (1988) Purification and reconstitution of the calcium release channel from skeletal muscle. Nature 331, 315–319 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

