A Molecular Basis for the Presentation of Phosphorylated Peptides by HLA-B Antigens
- PMID: 27920218
- PMCID: PMC5294207
- DOI: 10.1074/mcp.M116.063800
A Molecular Basis for the Presentation of Phosphorylated Peptides by HLA-B Antigens
Abstract
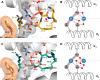
As aberrant protein phosphorylation is a hallmark of tumor cells, the display of tumor-specific phosphopeptides by Human Leukocyte Antigen (HLA) class I molecules can be exploited in the treatment of cancer by T-cell-based immunotherapy. Yet, the characterization and prediction of HLA-I phospholigands is challenging as the molecular determinants of the presentation of such post-translationally modified peptides are not fully understood. Here, we employed a peptidomic workflow to identify 256 unique phosphorylated ligands associated with HLA-B*40, -B*27, -B*39, or -B*07. Remarkably, these phosphopeptides showed similar molecular features. Besides the specific anchor motifs imposed by the binding groove of each allotype, the predominance of phosphorylation at peptide position 4 (P4) became strikingly evident, as was the enrichment of basic residues at P1. To determine the structural basis of this observation, we carried out a series of peptide binding assays and solved the crystal structures of HLA-B*40 in complex with a phosphorylated ligand or its nonphosphorylated counterpart. Overall, our data provide a clear explanation to the common motif found in the phosphopeptidomes associated to different HLA-B molecules. The high prevalence of phosphorylation at P4 is dictated by the presence of the conserved residue Arg62 in the heavy chain, a structural feature shared by most HLA-B alleles. In contrast, the preference for basic residues at P1 is allotype-dependent and might be linked to the structure of the A pocket. This molecular understanding of the presentation of phosphopeptides by HLA-B molecules provides a base for the improved prediction and identification of phosphorylated neo-antigens, as potentially used for cancer immunotherapy.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

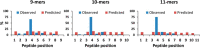



References
-
- Robinson J., Soormally A. R., Hayhurst J. D., and Marsh S. G. (2016) The IPD-IMGT/HLA Database - New developments in reporting HLA variation. Human Immunol. 77, 233–237 - PubMed
-
- Engelhard V. H., Altrich-Vanlith M., Ostankovitch M., and Zarling A. L. (2006) Post-translational modifications of naturally processed MHC-binding epitopes. Cur. Opin. Immunol. 18, 92–97 - PubMed
-
- Meyer V. S., Drews O., Gunder M., Hennenlotter J., Rammensee H. G., and Stevanovic S. (2009) Identification of natural MHC class II presented phosphopeptides and tumor-derived MHC class I phospholigands. J. Proteome Res. 8, 3666–3674 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

