Ischemic stroke disrupts the endothelial glycocalyx through activation of proHPSE via acrolein exposure
- PMID: 33127645
- PMCID: PMC7939480
- DOI: 10.1074/jbc.RA120.015105
Ischemic stroke disrupts the endothelial glycocalyx through activation of proHPSE via acrolein exposure
Abstract
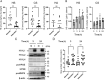
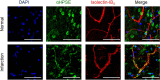
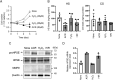
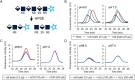
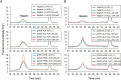
Infiltration of peripheral immune cells after blood-brain barrier dysfunction causes severe inflammation after a stroke. Although the endothelial glycocalyx, a network of membrane-bound glycoproteins and proteoglycans that covers the lumen of endothelial cells, functions as a barrier to circulating cells, the relationship between stroke severity and glycocalyx dysfunction remains unclear. In this study, glycosaminoglycans, a component of the endothelial glycocalyx, were studied in the context of ischemic stroke using a photochemically induced thrombosis mouse model. Decreased levels of heparan sulfate and chondroitin sulfate and increased activity of hyaluronidase 1 and heparanase (HPSE) were observed in ischemic brain tissues. HPSE expression in cerebral vessels increased after stroke onset and infarct volume greatly decreased after co-administration of N-acetylcysteine + glycosaminoglycan oligosaccharides as compared with N-acetylcysteine administration alone. These results suggest that the endothelial glycocalyx was injured after the onset of stroke. Interestingly, scission activity of proHPSE produced by immortalized endothelial cells and HEK293 cells transfected with hHPSE1 cDNA were activated by acrolein (ACR) exposure. We identified the ACR-modified amino acid residues of proHPSE using nano LC-MS/MS, suggesting that ACR modification of Lys139 (6-kDa linker), Lys107, and Lys161, located in the immediate vicinity of the 6-kDa linker, at least in part is attributed to the activation of proHPSE. Because proHPSE, but not HPSE, localizes outside cells by binding with heparan sulfate proteoglycans, ACR-modified proHPSE represents a promising target to protect the endothelial glycocalyx.
Keywords: acrolein; chondroitin sulfate; glycocalyx; heparan sulfate; heparanase; hyaluronan; hyaluronidase; ischemic stroke; stroke.
© 2020 Ko et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Zhang J., Takahashi H.K., Liu K., Wake H., Liu R., Maruo T., Date I., Yoshino T., Ohtsuka A., Mori S., Nishibori M. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42:1420–1428. doi: 10.1161/STROKEAHA.110.598334. 21474801. - DOI - PubMed
-
- Shichita T., Hasegawa E., Kimura A., Morita R., Sakaguchi R., Takada I., Sekiya T., Ooboshi H., Kitazono T., Yanagawa T., Ishii T., Takahashi H., Mori S., Nishibori M., Kuroda K. Peroxiredoxin family proteins are key initiators of post-ischemic inflammation in the brain. Nat. Med. 2012;18:911–917. doi: 10.1038/nm.2749. 22610280. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

