Electron Accepting Units of the Diheme Cytochrome c TsdA, a Bifunctional Thiosulfate Dehydrogenase/Tetrathionate Reductase
- PMID: 27694441
- PMCID: PMC5122753
- DOI: 10.1074/jbc.M116.753863
Electron Accepting Units of the Diheme Cytochrome c TsdA, a Bifunctional Thiosulfate Dehydrogenase/Tetrathionate Reductase
Abstract
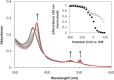
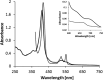
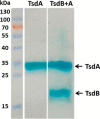
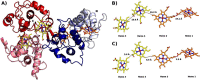
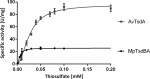
The enzymes of the thiosulfate dehydrogenase (TsdA) family are wide-spread diheme c-type cytochromes. Here, redox carriers were studied mediating the flow of electrons arising from thiosulfate oxidation into respiratory or photosynthetic electron chains. In a number of organisms, including Thiomonas intermedia and Sideroxydans lithotrophicus, the tsdA gene is immediately preceded by tsdB encoding for another diheme cytochrome. Spectrophotometric experiments in combination with enzymatic assays in solution showed that TsdB acts as an effective electron acceptor of TsdA in vitro when TsdA and TsdB originate from the same source organism. Although TsdA covers a range from -300 to +150 mV, TsdB is redox active between -100 and +300 mV, thus enabling electron transfer between these hemoproteins. The three-dimensional structure of the TsdB-TsdA fusion protein from the purple sulfur bacterium Marichromatium purpuratum was solved by X-ray crystallography to 2.75 Å resolution providing insights into internal electron transfer. In the oxidized state, this tetraheme cytochrome c contains three hemes with axial His/Met ligation, whereas heme 3 exhibits the His/Cys coordination typical for TsdA active sites. Interestingly, thiosulfate is covalently bound to Cys330 on heme 3. In several bacteria, including Allochromatium vinosum, TsdB is not present, precluding a general and essential role for electron flow. Both AvTsdA and the MpTsdBA fusion react efficiently in vitro with high potential iron-sulfur protein from A. vinosum (Em +350 mV). High potential iron-sulfur protein not only acts as direct electron donor to the reaction center in anoxygenic phototrophs but can also be involved in aerobic respiratory chains.
Keywords: TsdA; crystal structure; cytochrome c; electron acceptor; enzyme kinetics; heme; protein chemistry; respiratory chain; thiosulfate dehydrogenase.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Denkmann K., Grein F., Zigann R., Siemen A., Bergmann J., van Helmont S., Nicolai A., Pereira I. A., and Dahl C. (2012) Thiosulfate dehydrogenase: a widespread unusual acidophilic c-type cytochrome. Environ. Microbiol. 14, 2673–2688 - PubMed
-
- Pires R. H., Venceslau S. S., Morais F., Teixeira M., Xavier A. V., and Pereira I. A. (2006) Characterization of the Desulfovibrio desulfuricans ATCC 27774 DsrMKJOP complex–a membrane-bound redox complex involved in the sulfate respiratory pathway. Biochemistry 45, 249–262 - PubMed
-
- Reijerse E. J., Sommerhalter M., Hellwig P., Quentmeier A., Rother D., Laurich C., Bothe E., Lubitz W., and Friedrich C. G. (2007) The unusual redox centers of SoxXA, a novel c-type heme-enzyme essential for chemotrophic sulfur-oxidation of Paracoccus pantotrophus. Biochemistry 46, 7804–7810 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

