A carbohydrate-binding family 48 module enables feruloyl esterase action on polymeric arabinoxylan
- PMID: 31558605
- PMCID: PMC6873179
- DOI: 10.1074/jbc.RA119.009523
A carbohydrate-binding family 48 module enables feruloyl esterase action on polymeric arabinoxylan
Abstract
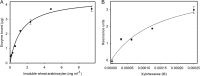
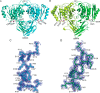
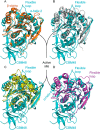
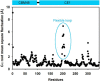
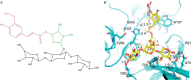
Feruloyl esterases (EC 3.1.1.73), belonging to carbohydrate esterase family 1 (CE1), hydrolyze ester bonds between ferulic acid (FA) and arabinose moieties in arabinoxylans. Recently, some CE1 enzymes identified in metagenomics studies have been predicted to contain a family 48 carbohydrate-binding module (CBM48), a CBM family associated with starch binding. Two of these CE1s, wastewater treatment sludge (wts) Fae1A and wtsFae1B isolated from wastewater treatment surplus sludge, have a cognate CBM48 domain and are feruloyl esterases, and wtsFae1A binds arabinoxylan. Here, we show that wtsFae1B also binds to arabinoxylan and that neither binds starch. Surface plasmon resonance analysis revealed that wtsFae1B's Kd for xylohexaose is 14.8 μm and that it does not bind to starch mimics, β-cyclodextrin, or maltohexaose. Interestingly, in the absence of CBM48 domains, the CE1 regions from wtsFae1A and wtsFae1B did not bind arabinoxylan and were also unable to catalyze FA release from arabinoxylan. Pretreatment with a β-d-1,4-xylanase did enable CE1 domain-mediated FA release from arabinoxylan in the absence of CBM48, indicating that CBM48 is essential for the CE1 activity on the polysaccharide. Crystal structures of wtsFae1A (at 1.63 Å resolution) and wtsFae1B (1.98 Å) revealed that both are folded proteins comprising structurally-conserved hydrogen bonds that lock the CBM48 position relative to that of the CE1 domain. wtsFae1A docking indicated that both enzymes accommodate the arabinoxylan backbone in a cleft at the CE1-CBM48 domain interface. Binding at this cleft appears to enable CE1 activities on polymeric arabinoxylan, illustrating an unexpected and crucial role of CBM48 domains for accommodating arabinoxylan.
Keywords: arabinoxylan; carbohydrate esterase family 1; carbohydrate-binding module; crystal structure; enzyme catalysis; enzyme mechanism; ferulic acid esterase; molecular docking; molecular dynamics; structure–function.
© 2019 Holck et al.
Conflict of interest statement
J. B. and K. B. R. M. K. are employees at Novozymes A/S, a company that produces and sells enzymes and microbes for industrial uses
Figures




References
-
- Garg S. (2016) Xylanase: applications in biofuel production. Curr. Metabolomics 4, 23–37 10.2174/2213235X03666150915211224 - DOI
-
- González-García S., Morales P. C., and Gullón B. (2018) Estimating the environmental impacts of a brewery waste-based biorefinery: bio-ethanol and xylooligosaccharides joint production case study. Ind. Crops Prod. 123, 331–340 10.1016/j.indcrop.2018.07.003 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

