The crystal structures of a chloride-pumping microbial rhodopsin and its proton-pumping mutant illuminate proton transfer determinants
- PMID: 32703899
- PMCID: PMC7606686
- DOI: 10.1074/jbc.RA120.014118
The crystal structures of a chloride-pumping microbial rhodopsin and its proton-pumping mutant illuminate proton transfer determinants
Abstract
Microbial rhodopsins are versatile and ubiquitous retinal-binding proteins that function as light-driven ion pumps, light-gated ion channels, and photosensors, with potential utility as optogenetic tools for altering membrane potential in target cells. Insights from crystal structures have been central for understanding proton, sodium, and chloride transport mechanisms of microbial rhodopsins. Two of three known groups of anion pumps, the archaeal halorhodopsins (HRs) and bacterial chloride-pumping rhodopsins, have been structurally characterized. Here we report the structure of a representative of a recently discovered third group consisting of cyanobacterial chloride and sulfate ion-pumping rhodopsins, the Mastigocladopsis repens rhodopsin (MastR). Chloride-pumping MastR contains in its ion transport pathway a unique Thr-Ser-Asp (TSD) motif, which is involved in the binding of a chloride ion. The structure reveals that the chloride-binding mode is more similar to HRs than chloride-pumping rhodopsins, but the overall structure most closely resembles bacteriorhodopsin (BR), an archaeal proton pump. The MastR structure shows a trimer arrangement reminiscent of BR-like proton pumps and shows features at the extracellular side more similar to BR than the other chloride pumps. We further solved the structure of the MastR-T74D mutant, which contains a single amino acid replacement in the TSD motif. We provide insights into why this point mutation can convert the MastR chloride pump into a proton pump but cannot in HRs. Our study points at the importance of precise coordination and exact location of the water molecule in the active center of proton pumps, which serves as a bridge for the key proton transfer.
Keywords: Mastigocladopsis repens; X-ray crystallography; bacteriorhodopsin; bicelle crystallization; chloride pump; chloride transport; cyanobacterial rhodopsin; functional conversion; functional interconversion; membrane protein; microbial rhodopsin; protein structure; proton pump; retinal protein; structure–function.
© 2020 Besaw et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures



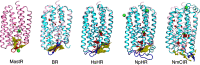


References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

