Effects of α-tubulin acetylation on microtubule structure and stability
- PMID: 31072936
- PMCID: PMC6535015
- DOI: 10.1073/pnas.1900441116
Effects of α-tubulin acetylation on microtubule structure and stability
Abstract
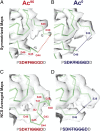
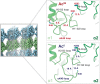
Acetylation of K40 in α-tubulin is the sole posttranslational modification to mark the luminal surface of microtubules. It is still controversial whether its relationship with microtubule stabilization is correlative or causative. We have obtained high-resolution cryo-electron microscopy (cryo-EM) reconstructions of pure samples of αTAT1-acetylated and SIRT2-deacetylated microtubules to visualize the structural consequences of this modification and reveal its potential for influencing the larger assembly properties of microtubules. We modeled the conformational ensembles of the unmodified and acetylated states by using the experimental cryo-EM density as a structural restraint in molecular dynamics simulations. We found that acetylation alters the conformational landscape of the flexible loop that contains αK40. Modification of αK40 reduces the disorder of the loop and restricts the states that it samples. We propose that the change in conformational sampling that we describe, at a location very close to the lateral contacts site, is likely to affect microtubule stability and function.
Keywords: MD; acetylation; cryo-EM; microtubule; tubulin modifications.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: E.N. and C.J. were coauthors in the 2016 review article “Microtubules: 50 Years on from the discovery of tubulin” [Borisy G, et al. (1)].
Figures


References
-
- Nogales E, Whittaker M, Milligan RA, Downing KH (1999) High-resolution model of the microtubule. Cell 96:79–88. - PubMed
-
- Mitchison TJ. (1993) Localization of an exchangeable GTP binding site at the plus end of microtubules. Science 261:1044–1047. - PubMed
-
- Mitchison T, Kirschner M (1984) Dynamic instability of microtubule growth. Nature 312:237–242. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

