Inhibition of strigolactone receptors by N-phenylanthranilic acid derivatives: Structural and functional insights
- PMID: 29523686
- PMCID: PMC5925799
- DOI: 10.1074/jbc.RA117.001154
Inhibition of strigolactone receptors by N-phenylanthranilic acid derivatives: Structural and functional insights
Abstract
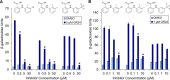
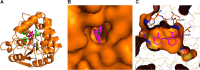
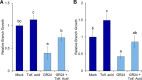
The strigolactone (SL) family of plant hormones regulates a broad range of physiological processes affecting plant growth and development and also plays essential roles in controlling interactions with parasitic weeds and symbiotic fungi. Recent progress elucidating details of SL biosynthesis, signaling, and transport offers many opportunities for discovering new plant-growth regulators via chemical interference. Here, using high-throughput screening and downstream biochemical assays, we identified N-phenylanthranilic acid derivatives as potent inhibitors of the SL receptors from petunia (DAD2), rice (OsD14), and Arabidopsis (AtD14). Crystal structures of DAD2 and OsD14 in complex with inhibitors further provided detailed insights into the inhibition mechanism, and in silico modeling of 19 other plant strigolactone receptors suggested that these compounds are active across a large range of plant species. Altogether, these results provide chemical tools for investigating SL signaling and further define a framework for structure-based approaches to design and validate optimized inhibitors of SL receptors for specific plant targets.
Keywords: chemical biology; crystal structure; hormone receptor; hydrolase; inhibition mechanism; plant hormone; strigolactone; strigolactone receptor inhibitor.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
C. H., K. C. S., and the New Zealand Institute for Plant and Food Research Limited have filed a New Zealand provisional patent application (734620) that relates to the specific topic “Strigolactone receptor antagonists.”
Figures





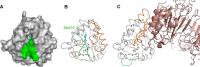
References
-
- Gomez-Roldan V., Fermas S., Brewer P. B., Puech-Pagès V., Dun E. A., Pillot J.-P., Letisse F., Matusova R., Danoun S., Portais J.-C., Bouwmeester H., Becard G., Beveridge C. A., Rameau C., and Rochange S. F. (2008) Strigolactone inhibition of shoot branching. Nature 455, 189–194 10.1038/nature07271 - DOI - PubMed
-
- Kapulnik Y., Delaux P. M., Resnick N., Mayzlish-Gati E., Wininger S., Bhattacharya C., Séjalon-Delmas N., Combier J. P., Bécard G., Belausov E., Beeckman T., Dor E., Hershenhorn J., and Koltai H. (2011) Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 233, 209–216 10.1007/s00425-010-1310-y - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

