Structure-function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating
- PMID: 30139744
- PMCID: PMC6187627
- DOI: 10.1074/jbc.RA118.005066
Structure-function analyses of the ion channel TRPC3 reveal that its cytoplasmic domain allosterically modulates channel gating
Abstract
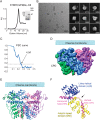
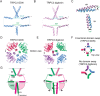
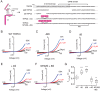
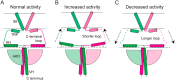
The transient receptor potential ion channels support Ca2+ permeation in many organs, including the heart, brain, and kidney. Genetic mutations in transient receptor potential cation channel subfamily C member 3 (TRPC3) are associated with neurodegenerative diseases, memory loss, and hypertension. To better understand the conformational changes that regulate TRPC3 function, we solved the cryo-EM structures for the full-length human TRPC3 and its cytoplasmic domain (CPD) in the apo state at 5.8- and 4.0-Å resolution, respectively. These structures revealed that the TRPC3 transmembrane domain resembles those of other TRP channels and that the CPD is a stable module involved in channel assembly and gating. We observed the presence of a C-terminal domain swap at the center of the CPD where horizontal helices (HHs) transition into a coiled-coil bundle. Comparison of TRPC3 structures revealed that the HHs can reside in two distinct positions. Electrophysiological analyses disclosed that shortening the length of the C-terminal loop connecting the HH with the TRP helices increases TRPC3 activity and that elongating the length of the loop has the opposite effect. Our findings indicate that the C-terminal loop affects channel gating by altering the allosteric coupling between the cytoplasmic and transmembrane domains. We propose that molecules that target the HH may represent a promising strategy for controlling TRPC3-associated neurological disorders and hypertension.
Keywords: GSK-1702934A; TRPC3; calcium channel; cryo-electron microscopy; electrophysiology; ion channel; neurotransmitter; structural biology; transient receptor potential channels (TRP channels); vascular biology.
© 2018 Sierra-Valdez et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Bröker-Lai J., Kollewe A., Schindeldecker B., Pohle J., Nguyen Chi V., Mathar I., Guzman R., Schwarz Y., Lai A., Weißgerber P., Schwegler H., Dietrich A., Both M., Sprengel R., Draguhn A., et al. (2017) Heteromeric channels formed by TRPC1, TRPC4 and TRPC5 define hippocampal synaptic transmission and working memory. EMBO J. 36, 2770–2789 10.15252/embj.201696369 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

