Functional genomics of epilepsy-associated mutations in the GABAA receptor subunits reveal that one mutation impairs function and two are catastrophic
- PMID: 30728247
- PMCID: PMC6463728
- DOI: 10.1074/jbc.RA118.005697
Functional genomics of epilepsy-associated mutations in the GABAA receptor subunits reveal that one mutation impairs function and two are catastrophic
Abstract
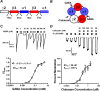
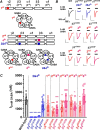
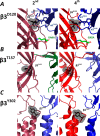
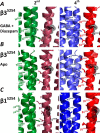
A number of epilepsy-causing mutations have recently been identified in the genes of the α1, β3, and γ2 subunits comprising the γ-aminobutyric acid type A (GABAA) receptor. These mutations are typically dominant, and in certain cases, such as the α1 and β3 subunits, they may lead to a mix of receptors at the cell surface that contain no mutant subunits, a single mutated subunit, or two mutated subunits. To determine the effects of mutations in a single subunit or in two subunits on receptor activation, we created a concatenated protein assembly that links all five subunits of the α1β3γ2 receptor and expresses them in the correct orientation. We created nine separate receptor variants with a single-mutant subunit and four receptors containing two subunits of the γ2R323Q, β3D120N, β3T157M, β3Y302C, and β3S254F epilepsy-causing mutations. We found that the singly mutated γ2R323Q subunit impairs GABA activation of the receptor by reducing GABA potency. A single β3D120N, β3T157M, or β3Y302C mutation also substantially impaired receptor activation, and two copies of these mutants within a receptor were catastrophic. Of note, an effect of the β3S254F mutation on GABA potency depended on the location of this mutant subunit within the receptor, possibly because of the membrane environment surrounding the transmembrane region of the receptor. Our results highlight that precise functional genomic analyses of GABAA receptor mutations using concatenated constructs can identify receptors with an intermediate phenotype that contribute to epileptic phenotypes and that are potential drug targets for precision medicine approaches.
Keywords: GABA receptor; brain disorder; channel activation; concatemer; cryo-electron microscopy; epilepsy; heterozygous; missense; neurotransmission; neurotransmitter; synaptic transmission.
© 2019 Absalom et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Hernandez C. C., Klassen T. L., Jackson L. G., Gurba K., Hu N., Noebels J. L., and Macdonald R. L. (2016) Deleterious rare variants reveal risk for loss of GABAA receptor function in patients with genetic epilepsy and in the general population. PLoS One 11, e0162883 10.1371/journal.pone.0162883 - DOI - PMC - PubMed
-
- Johannesen K., Marini C., Pfeffer S., Møller R. S., Dorn T., Niturad C. E., Gardella E., Weber Y., Søndergård M., Hjalgrim H., Nikanorova M., Becker F., Larsen L. H., Dahl H. A., Maier O., et al. (2016) Phenotypic spectrum of GABRA1: from generalized epilepsies to severe epileptic encephalopathies. Neurology 87, 1140–1151 10.1212/WNL.0000000000003087 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical

