The molecular basis of endolytic activity of a multidomain alginate lyase from Defluviitalea phaphyphila, a representative of a new lyase family, PL39
- PMID: 31624143
- PMCID: PMC6885643
- DOI: 10.1074/jbc.RA119.010716
The molecular basis of endolytic activity of a multidomain alginate lyase from Defluviitalea phaphyphila, a representative of a new lyase family, PL39
Abstract
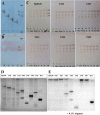
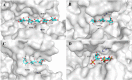
Alginate is a polymer containing two uronic acid epimers, β-d-mannuronate (M) and α-l-guluronate (G), and is a major component of brown seaweed that is depolymerized by alginate lyases. These enzymes have diverse specificity, cleaving the chain with endo- or exotype activity and with differential selectivity for the sequence of M or G at the cleavage site. Dp0100 is a 201-kDa multimodular, broad-specificity endotype alginate lyase from the marine thermophile Defluviitalea phaphyphila, which uses brown algae as a carbon source, converting it to ethanol, and bioinformatics analysis suggested that its catalytic domain represents a new polysaccharide lyase family, PL39. The structure of the Dp0100 catalytic domain, determined at 2.07 Å resolution, revealed that it comprises three regions strongly resembling those of the exotype lyase families PL15 and PL17. The conservation of key catalytic histidine and tyrosine residues belonging to the latter suggests these enzymes share mechanistic similarities. A complex of Dp0100 with a pentasaccharide, M5, showed that the oligosaccharide is located in subsites -2, -1, +1, +2, and +3 in a long, deep canyon open at both ends, explaining the endotype activity of this lyase. This contrasted with the hindered binding sites of the exotype enzymes, which are blocked such that only one sugar moiety can be accommodated at the -1 position in the catalytic site. The biochemical and structural analyses of Dp0100, the first for this new class of endotype alginate lyases, have furthered our understanding of the structure-function and evolutionary relationships within this important class of enzymes.
Keywords: alginate lyase; carbohydrate-binding protein; crystal structure; metalloenzyme; oligosaccharide; structure–function; substrate specificity.
© 2019 Ji et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

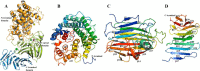




References
-
- Chung I. K., Beardall J., Mehta S., Sahoo D., and Stojkovic S. (2011) Using marine macroalgae for carbon sequestration: a critical appraisal. J. Appl. Phycol. 23, 877–886 10.1007/s10811-010-9604-9 - DOI
-
- Enquist-Newman M., Faust A. M., Bravo D. D., Santos C. N., Raisner R. M., Hanel A., Sarvabhowman P., Le C., Regitsky D. D., Cooper S. R., Peereboom L., Clark A., Martinez Y., Goldsmith J., Cho M. Y., et al. (2014) Efficient ethanol production from brown macroalgae sugars by a synthetic yeast platform. Nature 505, 239–243 10.1038/nature12771 - DOI - PubMed
-
- Wargacki A. J., Leonard E., Win M. N., Regitsky D. D., Santos C. N., Kim P. B., Cooper S. R., Raisner R. M., Herman A., Sivitz A. B., Lakshmanaswamy A., Kashiyama Y., Baker D., and Yoshikuni Y. (2012) An engineered microbial platform for direct biofuel production from brown macroalgae. Science 335, 308–313 10.1126/science.1214547 - DOI - PubMed
-
- Gacesa P. (1988) Alginates. Carbohyd. Polym. 8, 161–182 10.1016/0144-8617(88)90001-X - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

