Biochemical and structural insights into how amino acids regulate pyruvate kinase muscle isoform 2
- PMID: 32144209
- PMCID: PMC7170521
- DOI: 10.1074/jbc.RA120.013030
Biochemical and structural insights into how amino acids regulate pyruvate kinase muscle isoform 2
Abstract
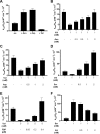
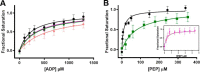
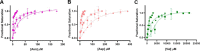
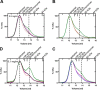
Pyruvate kinase muscle isoform 2 (PKM2) is a key glycolytic enzyme involved in ATP generation and critical for cancer metabolism. PKM2 is expressed in many human cancers and is regulated by complex mechanisms that promote tumor growth and proliferation. Therefore, it is considered an attractive therapeutic target for modulating tumor metabolism. Various stimuli allosterically regulate PKM2 by cycling it between highly active and less active states. Several small molecules activate PKM2 by binding to its intersubunit interface. Serine and cysteine serve as an activator and inhibitor of PKM2, respectively, by binding to its amino acid (AA)-binding pocket, which therefore represents a potential druggable site. Despite binding similarly to PKM2, how cysteine and serine differentially regulate this enzyme remains elusive. Using kinetic analyses, fluorescence binding, X-ray crystallography, and gel filtration experiments with asparagine, aspartate, and valine as PKM2 ligands, we examined whether the differences in the side-chain polarity of these AAs trigger distinct allosteric responses in PKM2. We found that Asn (polar) and Asp (charged) activate PKM2 and that Val (hydrophobic) inhibits it. The results also indicate that both Asn and Asp can restore the activity of Val-inhibited PKM2. AA-bound crystal structures of PKM2 displayed distinctive interactions within the binding pocket, causing unique allosteric effects in the enzyme. These structure-function analyses of AA-mediated PKM2 regulation shed light on the chemical requirements in the development of mechanism-based small-molecule modulators targeting the AA-binding pocket of PKM2 and provide broader insights into the regulatory mechanisms of complex allosteric enzymes.
Keywords: allosteric regulation; amino acids; asparagine; aspartate; cancer metabolism; crystal structure; enzyme kinetics; fluorescence binding; glycolysis; oligomerization; pyruvate kinase; pyruvate kinase muscle isoform 2 (PKM2); valine.
© 2020 Nandi and Dey.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Anastasiou D., Yu Y., Israelsen W. J., Jiang J. K., Boxer M. B., Hong B. S., Tempel W., Dimov S., Shen M., Jha A., Yang H., Mattaini K. R., Metallo C. M., Fiske B. P., Courtney K. D., et al. (2012) Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 8, 839–847 10.1038/nchembio.1060 - DOI - PMC - PubMed
-
- Yacovan A., Ozeri R., Kehat T., Mirilashvili S., Sherman D., Aizikovich A., Shitrit A., Ben-Zeev E., Schutz N., Bohana-Kashtan O., Konson A., Behar V., and Becker O. M. (2012) 1-(Sulfonyl)-5-(arylsulfonyl)indoline as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg. Med. Chem. Lett. 22, 6460–6468 10.1016/j.bmcl.2012.08.054 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

