The Vibrio cholerae minor pilin TcpB mediates uptake of the cholera toxin phage CTXφ
- PMID: 31471320
- PMCID: PMC6816102
- DOI: 10.1074/jbc.RA119.009980
The Vibrio cholerae minor pilin TcpB mediates uptake of the cholera toxin phage CTXφ
Abstract
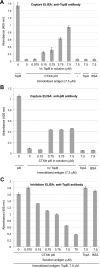
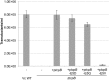
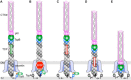
Virulent strains of the bacterial pathogen Vibrio cholerae cause the diarrheal disease cholera by releasing cholera toxin into the small intestine. V. cholerae acquired its cholera toxin genes by lysogenic infection with the filamentous bacteriophage CTXφ. CTXφ uses its minor coat protein pIII, located in multiple copies at the phage tip, to bind to the V. cholerae toxin-coregulated pilus (TCP). However, the molecular details of this interaction and the mechanism of phage internalization are not well-understood. The TCP filament is a polymer of major pilins, TcpA, and one or more minor pilin, TcpB. TCP are retractile, with both retraction and assembly initiated by TcpB. Consistent with these roles in pilus dynamics, we hypothesized that TcpB controls both binding and internalization of CTXφ. To test this hypothesis, we determined the crystal structure of the C-terminal half of TcpB and characterized its interactions with CTXφ pIII. We show that TcpB is a homotrimer in its crystallographic form as well as in solution and is present in multiple copies at the pilus tip, which likely facilitates polyvalent binding to pIII proteins at the phage tip. We further show that recombinant forms of TcpB and pIII interact in vitro, and both TcpB and anti-TcpB antibodies block CTXφ infection of V. cholerae Finally, we show that CTXφ uptake requires TcpB-mediated retraction. Our data support a model whereby CTXφ and TCP bind in a tip-to-tip orientation, allowing the phage to be drawn into the V. cholerae periplasm as an extension of the pilus filament.
Keywords: CTXφ; TcpB; Vibrio cholerae; X-ray crystallography; bacteriophage; minor pilins; pIII; protein structure; toxin coregulated pilus; type IV pili.
© 2019 Gutierrez-Rodarte et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

