Cryo-EM structure of the native rhodopsin dimer in nanodiscs
- PMID: 31399513
- PMCID: PMC6768649
- DOI: 10.1074/jbc.RA119.010089
Cryo-EM structure of the native rhodopsin dimer in nanodiscs
Abstract
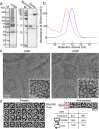
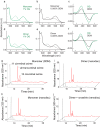
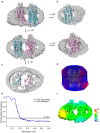
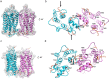
Imaging of rod photoreceptor outer-segment disc membranes by atomic force microscopy and cryo-electron tomography has revealed that the visual pigment rhodopsin, a prototypical class A G protein-coupled receptor (GPCR), can organize as rows of dimers. GPCR dimerization and oligomerization offer possibilities for allosteric regulation of GPCR activity, but the detailed structures and mechanism remain elusive. In this investigation, we made use of the high rhodopsin density in the native disc membranes and of a bifunctional cross-linker that preserves the native rhodopsin arrangement by covalently tethering rhodopsins via Lys residue side chains. We purified cross-linked rhodopsin dimers and reconstituted them into nanodiscs for cryo-EM analysis. We present cryo-EM structures of the cross-linked rhodopsin dimer as well as a rhodopsin dimer reconstituted into nanodiscs from purified monomers. We demonstrate the presence of a preferential 2-fold symmetrical dimerization interface mediated by transmembrane helix 1 and the cytoplasmic helix 8 of rhodopsin. We confirmed this dimer interface by double electron-electron resonance measurements of spin-labeled rhodopsin. We propose that this interface and the arrangement of two protomers is a prerequisite for the formation of the observed rows of dimers. We anticipate that the approach outlined here could be extended to other GPCRs or membrane receptors to better understand specific receptor dimerization mechanisms.
Keywords: G protein-coupled receptor (GPCR); cell signaling; cryo-electron microscopy (cryo-EM); double electron-electron resonance (DEER); helix 8 (H8); receptor; retina; retinoid-binding protein; rhodopsin; rhodopsin dimerization; rod outer segment; transducin; transmembrane helix 1 (TM1).
© 2019 Zhao et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions

