Structural insights into emergent signaling modes of G protein-coupled receptors
- PMID: 32571882
- PMCID: PMC7450137
- DOI: 10.1074/jbc.REV120.009348
Structural insights into emergent signaling modes of G protein-coupled receptors
Abstract
G protein-coupled receptors (GPCRs) represent the largest family of cell membrane proteins, with >800 GPCRs in humans alone, and recognize highly diverse ligands, ranging from photons to large protein molecules. Very important to human medicine, GPCRs are targeted by about 35% of prescription drugs. GPCRs are characterized by a seven-transmembrane α-helical structure, transmitting extracellular signals into cells to regulate major physiological processes via heterotrimeric G proteins and β-arrestins. Initially viewed as receptors whose signaling via G proteins is delimited to the plasma membrane, it is now recognized that GPCRs signal also at various intracellular locations, and the mechanisms and (patho)physiological relevance of such signaling modes are actively investigated. The propensity of GPCRs to adopt different signaling modes is largely encoded in the structural plasticity of the receptors themselves and of their signaling complexes. Here, we review emerging modes of GPCR signaling via endosomal membranes and the physiological implications of such signaling modes. We further summarize recent structural insights into mechanisms of GPCR activation and signaling. We particularly emphasize the structural mechanisms governing the continued GPCR signaling from endosomes and the structural aspects of the GPCR resensitization mechanism and discuss the recently uncovered and important roles of lipids in these processes.
Keywords: G protein-coupled receptor (GPCR); arrestin; cAMP; cyclic AMP (cAMP); endosomal membrane; endosome; environmental sensing; membrane lipid; parathyroid hormone; receptor structure-function; signal transduction; structural dynamics; structure-function; β-arrestins.
© 2020 Sutkeviciute and Vilardaga.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the content of this article.
Figures




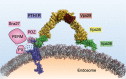
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

