A molecular roadmap to the plant immune system
- PMID: 32816993
- PMCID: PMC7606695
- DOI: 10.1074/jbc.REV120.010852
A molecular roadmap to the plant immune system
Abstract
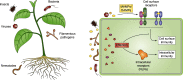
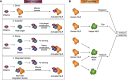
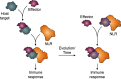
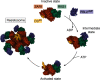
Plant diseases caused by pathogens and pests are a constant threat to global food security. Direct crop losses and the measures used to control disease (e.g. application of pesticides) have significant agricultural, economic, and societal impacts. Therefore, it is essential that we understand the molecular mechanisms of the plant immune system, a system that allows plants to resist attack from a wide variety of organisms ranging from viruses to insects. Here, we provide a roadmap to plant immunity, with a focus on cell-surface and intracellular immune receptors. We describe how these receptors perceive signatures of pathogens and pests and initiate immune pathways. We merge existing concepts with new insights gained from recent breakthroughs on the structure and function of plant immune receptors, which have generated a shift in our understanding of cell-surface and intracellular immunity and the interplay between the two. Finally, we use our current understanding of plant immunity as context to discuss the potential of engineering the plant immune system with the aim of bolstering plant defenses against disease.
Keywords: Nod-like receptor (NLR); cell surface receptor; cell-surface immunity; cellular immune response; effectors; intracellular immunity; nucleotide-binding leucine-rich repeat receptors (NLRs); plant biochemistry; plant defense; plant immunity; receptor-like kinases (RLKs); receptor-like proteins (RLPs); resistance engineering.
© 2020 Bentham et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Turner R. S. (2005) After the famine: Plant pathology, Phytophthora infestans, and the late blight of potatoes, 1845–1960. Hist. Stud. Phys. Biol. Sci. 35, 341–370 10.1525/hsps.2005.35.2.341 - DOI
-
- Dean R., Van Kan J. A., Pretorius Z. A., Hammond-Kosack K. E., Di Pietro A., Spanu P. D., Rudd J. J., Dickman M., Kahmann R., Ellis J., and Foster G. D. (2012) The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430 10.1111/j.1364-3703.2011.00783.x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

