Structural insights into the complex of oncogenic KRas4BG12V and Rgl2, a RalA/B activator
- PMID: 37833074
- PMCID: PMC10576006
- DOI: 10.26508/lsa.202302080
Structural insights into the complex of oncogenic KRas4BG12V and Rgl2, a RalA/B activator
Abstract
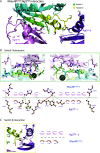
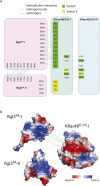
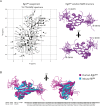
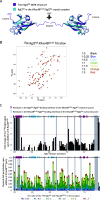
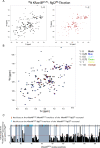
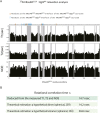
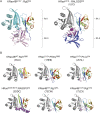
About a quarter of total human cancers carry mutations in Ras isoforms. Accumulating evidence suggests that small GTPases, RalA, and RalB, and their activators, Ral guanine nucleotide exchange factors (RalGEFs), play an essential role in oncogenic Ras-induced signalling. We studied the interaction between human KRas4B and the Ras association (RA) domain of Rgl2 (Rgl2RA), one of the RA-containing RalGEFs. We show that the G12V oncogenic KRas4B mutation changes the interaction kinetics with Rgl2RA The crystal structure of the KRas4BG12V: Rgl2RA complex shows a 2:2 heterotetramer where the switch I and switch II regions of each KRasG12V interact with both Rgl2RA molecules. This structural arrangement is highly similar to the HRasE31K:RALGDSRA crystal structure and is distinct from the well-characterised Ras:Raf complex. Interestingly, the G12V mutation was found at the dimer interface of KRas4BG12V with its partner. Our study reveals a potentially distinct mode of Ras:effector complex formation by RalGEFs and offers a possible mechanistic explanation for how the oncogenic KRas4BG12V hyperactivates the RalA/B pathway.
© 2023 Tariq et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures








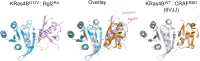



References
-
- Andreadelis I, Kiriakidi S, Lamprakis C, Theodoropoulou A, Doerr S, Chatzigoulas A, Manchester J, Velez-Vega C, Duca JS, Cournia Z (2022) Membrane composition and Raf[CRD]-Membrane attachment are driving forces for K-Ras4B dimer stability. J Phys Chem B 126: 1504–1519. 10.1021/acs.jpcb.1c01184 - DOI - PubMed
-
- Boucher W (2010) Azara, v2.8. Available at: http://www.cambridge2000.com/azara/
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
