The inhibitory action of the chaperone BRICHOS against the α-Synuclein secondary nucleation pathway
- PMID: 39567476
- PMCID: PMC11579453
- DOI: 10.1038/s41467-024-54212-2
The inhibitory action of the chaperone BRICHOS against the α-Synuclein secondary nucleation pathway
Abstract
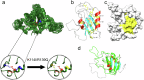
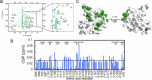
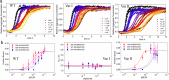
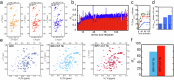
The complex kinetics of disease-related amyloid aggregation of proteins such as α-Synuclein (α-Syn) in Parkinson's disease and Aβ42 in Alzheimer's disease include primary nucleation, amyloid fibril elongation and secondary nucleation. The latter can be a key accelerator of the aggregation process. It has been demonstrated that the chaperone domain BRICHOS can interfere with the secondary nucleation process of Aβ42. Here, we explore the mechanism of secondary nucleation inhibition of the BRICHOS domain of the lung surfactant protein (proSP-C) against α-Syn aggregation and amyloid formation. We determine the 3D NMR structure of an inactive trimer of proSP-C BRICHOS and its active monomer using a designed mutant. Furthermore, the interaction between the proSP-C BRICHOS chaperone and a substrate peptide has been studied. NMR-based interaction studies of proSP-C BRICHOS with α-Syn fibrils show that proSP-C BRICHOS binds to the C-terminal flexible fuzzy coat of the fibrils, which is the secondary nucleation site on the fibrils. Super-resolution fluorescence microscopy demonstrates that proSP-C BRICHOS runs along the fibrillar axis diffusion-dependently sweeping off monomeric α-Syn from the fibrils. The observed mechanism explains how a weakly binding chaperone can inhibit the α-Syn secondary nucleation pathway via avidity where a single proSP-C BRICHOS molecule is sufficient against up to ~7-40 α-Syn molecules embedded within the fibrils.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interest.
Figures



References
-
- Spillantini, M. G. et al. Alpha-synuclein in Lewy bodies. Nature388, 839–40 (1997). - PubMed
-
- Krüger, R. et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat. Genet18, 106–8 (1998). - PubMed
-
- Ibáñez, P. et al. Causal relation between alpha-synuclein gene duplication and familial Parkinson’s disease. Lancet364, 1169–71 (2004). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

