Integrating fragment-based screening with targeted protein degradation and genetic rescue to explore eIF4E function
- PMID: 40016190
- PMCID: PMC11868579
- DOI: 10.1038/s41467-024-54356-1
Integrating fragment-based screening with targeted protein degradation and genetic rescue to explore eIF4E function
Abstract
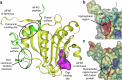
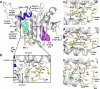
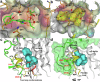
Eukaryotic initiation factor 4E (eIF4E) serves as a regulatory hub for oncogene-driven protein synthesis and is considered a promising anticancer target. Here we screen a fragment library against eIF4E and identify a ligand-binding site with previously unknown function. Follow-up structure-based design yields a low nM tool compound (4, Kd = 0.09 µM; LE 0.38), which disrupts the eIF4E:eIF4G interaction, inhibits translation in cell lysates, and demonstrates target engagement with eIF4E in intact cells (EC50 = 2 µM). By coupling targeted protein degradation with genetic rescue using eIF4E mutants, we show that disruption of both the canonical eIF4G and non-canonical binding sites is likely required to drive a strong cellular effect. This work highlights the power of fragment-based drug discovery to identify pockets in difficult-to-drug proteins and how this approach can be combined with genetic characterization and degrader technology to probe protein function in complex biological systems.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: S.Y.S., S.D.A., M.M., C.I.M., K.S., M.V.P., and P.A.C. are current or previous employees of The Institute of Cancer Research, which has a commercial interest in a range of drug targets and operates a Rewards to Discoverers scheme, through which employees may receive financial benefits following the commercial licensing of a project. A.J.W., C.J.R., M.G.C., B.D.C., C.E.E., E.C., J.C., S.D.H., C.M.F., P.N.M., N.P., P.P., S.M.S., J.S.D., M.V., Ge.W., H.W., and G.l.W. are current or previous employees of Astex Pharmaceuticals which has a commercial interest in a range of drug targets.
Figures

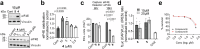



References
-
- Silvera, D., Formenti, S. C. & Schneider, R. J. Translational control in cancer. Nat. Rev. Cancer10, 254–266 (2010). - PubMed
-
- Fabbri, L., Chakraborty, A., Robert, C. & Vagner, S. The plasticity of mRNA translation during cancer progression and therapy resistance. Nat. Rev. Cancer21, 558–577 (2021). - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

