Crystal structure of the extracellular segment of integrin alpha Vbeta3
- PMID: 11546839
- PMCID: PMC2885948
- DOI: 10.1126/science.1064535
Crystal structure of the extracellular segment of integrin alpha Vbeta3
Abstract
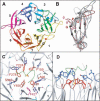
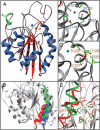
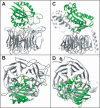
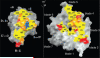
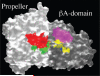
Integrins are alphabeta heterodimeric receptors that mediate divalent cation-dependent cell-cell and cell-matrix adhesion through tightly regulated interactions with ligands. We have solved the crystal structure of the extracellular portion of integrin alphaVbeta3 at 3.1 A resolution. Its 12 domains assemble into an ovoid "head" and two "tails." In the crystal, alphaVbeta3 is severely bent at a defined region in its tails, reflecting an unusual flexibility that may be linked to integrin regulation. The main inter-subunit interface lies within the head, between a seven-bladed beta-propeller from alphaV and an A domain from beta3, and bears a striking resemblance to the Galpha/Gbeta interface in G proteins. A metal ion-dependent adhesion site (MIDAS) in the betaA domain is positioned to participate in a ligand-binding interface formed of loops from the propeller and betaA domains. MIDAS lies adjacent to a calcium-binding site with a potential regulatory function.
Figures

Comment in
-
Structure. An anthropomorphic integrin.Science. 2001 Oct 12;294(5541):316-7. doi: 10.1126/science.1066240. Science. 2001. PMID: 11598288 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

