Extracellular chloride signals collagen IV network assembly during basement membrane formation
- PMID: 27216258
- PMCID: PMC4878091
- DOI: 10.1083/jcb.201510065
Extracellular chloride signals collagen IV network assembly during basement membrane formation
Abstract
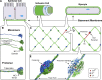
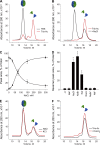
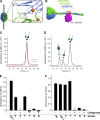
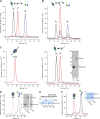
Basement membranes are defining features of the cellular microenvironment; however, little is known regarding their assembly outside cells. We report that extracellular Cl(-) ions signal the assembly of collagen IV networks outside cells by triggering a conformational switch within collagen IV noncollagenous 1 (NC1) domains. Depletion of Cl(-) in cell culture perturbed collagen IV networks, disrupted matrix architecture, and repositioned basement membrane proteins. Phylogenetic evidence indicates this conformational switch is a fundamental mechanism of collagen IV network assembly throughout Metazoa. Using recombinant triple helical protomers, we prove that NC1 domains direct both protomer and network assembly and show in Drosophila that NC1 architecture is critical for incorporation into basement membranes. These discoveries provide an atomic-level understanding of the dynamic interactions between extracellular Cl(-) and collagen IV assembly outside cells, a critical step in the assembly and organization of basement membranes that enable tissue architecture and function. Moreover, this provides a mechanistic framework for understanding the molecular pathobiology of NC1 domains.
© 2016 Cummings et al.
Figures


References
-
- Bhave G., Cummings C.F., Vanacore R.M., Kumagai-Cresse C., Ero-Tolliver I.A., Rafi M., Kang J.S., Pedchenko V., Fessler L.I., Fessler J.H., and Hudson B.G.. 2012. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat. Chem. Biol. 8:784–790. 10.1038/nchembio.1038 - DOI - PMC - PubMed
-
- Boutaud A., Borza D.B., Bondar O., Gunwar S., Netzer K.O., Singh N., Ninomiya Y., Sado Y., Noelken M.E., and Hudson B.G.. 2000. Type IV collagen of the glomerular basement membrane. Evidence that the chain specificity of network assembly is encoded by the noncollagenous NC1 domains. J. Biol. Chem. 275:30716–30724. 10.1074/jbc.M004569200 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- F30 DK100094/DK/NIDDK NIH HHS/United States
- P01 DK065123/DK/NIDDK NIH HHS/United States
- R25 DK096999/DK/NIDDK NIH HHS/United States
- R01 DK018381/DK/NIDDK NIH HHS/United States
- R01 DK095761/DK/NIDDK NIH HHS/United States
- R01 DK099467/DK/NIDDK NIH HHS/United States
- I01 BX002025/BX/BLRD VA/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
- R01 DK065138/DK/NIDDK NIH HHS/United States
- T32 HL094296/HL/NHLBI NIH HHS/United States
- T32 GM007347/GM/NIGMS NIH HHS/United States
- K08 DK097306/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

