Propofol versus placebo (with rescue with ketamine) before less invasive surfactant administration: study protocol for a multicenter, double-blind, placebo controlled trial (PROLISA)
- PMID: 32384914
- PMCID: PMC7206779
- DOI: 10.1186/s12887-020-02112-x
Propofol versus placebo (with rescue with ketamine) before less invasive surfactant administration: study protocol for a multicenter, double-blind, placebo controlled trial (PROLISA)
Abstract
Background: One major limitation for less invasive surfactant administration (LISA) is the difficulty in providing sedation before this procedure and the competitive risk of respiratory depression versus avoidance of intubation for most sedative or analgesic drugs used in this context. The objective of this study is to compare the need for mechanical ventilation within 72 h of life following premedication with propofol, versus placebo (rescue with ketamine), for the LISA procedure in preterm neonates born before 32 weeks gestational age (wGA).
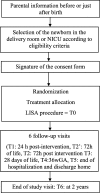
Methods: ProLISA is a phase III, non-inferiority, multicenter, double blind, randomized, placebo controlled trial designed according to the SPIRIT Statement. Neonates born before 32 wGA in 12 geographically dispersed Neonatal Intensive Care Units in France needing surfactant will be included from September 2019 to September 2022. A sample of 542 patients is needed. The neonate is randomized to the intervention (propofol) or control placebo group. Open label rescue treatment with ketamine is possible in both groups if FANS (Faceless Acute Neonatal pain Scale) is ≥6. To guide drug administration, FANS is scored before attempting laryngoscopy. Once an adequate score has been obtained, LISA is performed according to a standardized protocol. The primary outcome is the need for mechanical ventilation within 72 h of life. Secondary outcomes are tolerance of the procedure, pain evaluation, hemodynamic and neurologic parameters after the intervention, morbidities before discharge and neurodevelopmental assessment at 2 years of age.
Discussion: This paper describes the first multicenter, double-blind, randomized, placebo-controlled trial on this topic and will provide crucial information to support implementation of the LISA procedure.
Trial registration: ClinicalTrials.gov: NCT04016246. Registered 06 June 2019, N°EUDRACT: 2018-002876-41.
Keywords: Ketamine; Less invasive surfactant administration; Propofol; Randomized study; Sedation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Jensen EA, DeMauro SB, Kornhauser M, Aghai ZH, Greenspan JS, Dysart KC. Effects of multiple ventilation courses and duration of mechanical ventilation on respiratory outcomes in extremely low-birth-weight infants. JAMA Pediatr. 2015;169:1011–1017. doi: 10.1001/jamapediatrics.2015.2401. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical