Efficacy and Safety of Masitinib in Progressive Forms of Multiple Sclerosis: A Randomized, Phase 3, Clinical Trial
- PMID: 35190477
- PMCID: PMC9005047
- DOI: 10.1212/NXI.0000000000001148
Efficacy and Safety of Masitinib in Progressive Forms of Multiple Sclerosis: A Randomized, Phase 3, Clinical Trial
Abstract
Background and objectives: Masitinib is a selective tyrosine kinase inhibitor, targeting innate immune cells (mast cells and microglia) that are involved in the pathophysiology of progressive multiple sclerosis (MS). Study AB07002 assessed oral masitinib in patients with progressive MS who were progressing but not clinically active.
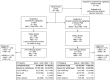
Methods: This randomized, double-blind, 2 parallel-group, placebo-controlled trial assessing 2 dose levels of masitinib vs equivalent placebo was conducted at 116 hospital clinics and specialized MS centers in 20 countries. Randomization (2:1) with minimization was performed centrally using an automated system. Patients, physicians, and outcome assessors remained masked to treatment group allocation. Patients with primary progressive MS (PPMS) or nonactive secondary progressive MS (nSPMS) without relapse for ≥2 years, aged 18-75 years, with baseline Expanded Disability Status Scale (EDSS) 2.0-6.0, and regardless of time from onset were treated for 96 weeks. The primary end point was overall EDSS change from baseline using repeated measures (generalized estimating equation, timeframe W12-W96, measured every 12 weeks), with positive values indicating increased clinical deterioration. Efficacy and safety were assessed in all randomly assigned and treated patients.
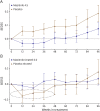
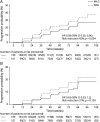
Results: A total of 611 patients were randomized; 301 in the masitinib 4.5 mg/kg/d parallel group and 310 in the uptitrated masitinib 6.0 mg/kg/d parallel group. Masitinib (4.5 mg/kg/d) (n = 199) showed significant benefit over placebo (n = 101) according to the primary end point, 0.001 vs 0.098, respectively, with a between-group difference of -0.097 (97% CI -0.192 to -0.002); p = 0.0256. Safety was consistent with masitinib's known profile (diarrhea, nausea, rash, and hematologic events), with no elevated risk of infection. Efficacy results from the independent uptitrated masitinib 6.0 mg/kg/d parallel group were inconclusive, and no new safety signal was observed.
Discussion: Masitinib (4.5 mg/kg/d) can benefit people with PPMS and nSPMS. A confirmatory phase 3 study will be initiated to substantiate these data.
Trial registration information: The first participant was randomized to study AB07002 on August 25, 2011. The trial was registered with the European Clinical Trials Database (#EudraCT 2010-021219-17) on July 1, 2011 (clinicaltrialsregister.eu/ctr-search/trial/2010-021219-17/ES) and with ClinicalTrials.gov (#NCT01433497) on September 14, 2011 (clinicaltrials.gov/ct2/show/NCT01433497).
Classification of evidence: This study provides Class II evidence that masitinib 4.5 mg/kg/d decreased progression of disability, measured by the EDSS, in adults with PPMS or patients with nSPMS (with no exacerbations in the last 2 years).
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907-911. - PubMed
-
- Stys PK, Tsutsui S. Recent advances in understanding multiple sclerosis. F1000Res. 2019;8:F1000 Faculty Rev-2100. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
