NeuroEPO plus (NeuralCIM®) in mild-to-moderate Alzheimer's clinical syndrome: the ATHENEA randomized clinical trial
- PMID: 38093366
- PMCID: PMC10716956
- DOI: 10.1186/s13195-023-01356-w
NeuroEPO plus (NeuralCIM®) in mild-to-moderate Alzheimer's clinical syndrome: the ATHENEA randomized clinical trial
Abstract
Background: NeuroEPO plus is a recombinant human erythropoietin without erythropoietic activity and shorter plasma half-life due to its low sialic acid content. NeuroEPO plus prevents oxidative damage, neuroinflammation, apoptosis and cognitive deficit in an Alzheimer's disease (AD) models. The aim of this study was to assess efficacy and safety of neuroEPO plus.
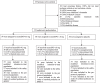
Methods: This was a double-blind, randomized, placebo-controlled, phase 2-3 trial involving participants ≥ 50 years of age with mild-to-moderate AD clinical syndrome. Participants were randomized in a 1:1:1 ratio to receive 0.5 or 1.0 mg of neuroEPO plus or placebo intranasally 3 times/week for 48 weeks. The primary outcome was change in the 11-item cognitive subscale of the AD Assessment Scale (ADAS-Cog11) score from baseline to 48 weeks (range, 0 to 70; higher scores indicate greater impairment). Secondary outcomes included CIBIC+, GDS, MoCA, NPI, Activities of Daily Living Scales, cerebral perfusion, and hippocampal volume.
Results: A total of 174 participants were enrolled and 170 were treated (57 in neuroEPO plus 0.5 mg, 56 in neuroEPO plus 1.0 mg and 57 in placebo group). Mean age, 74.0 years; 121 (71.2%) women and 85% completed the trial. The median change in ADAS-Cog11 score at 48 weeks was -3.0 (95% CI, -4.3 to -1.7) in the 0.5 mg neuroEPO plus group, -4.0 (95% CI, -5.9 to -2.1) in the 1.0 mg neuroEPO plus group and 4.0 (95% CI, 1.9 to 6.1) in the placebo group. The difference of neuroEPO plus 0.5 mg vs. placebo was 7.0 points (95% CI, 4.5-9.5) P = 0.000 and between the neuroEPO plus 1.0 mg vs. placebo was 8.0 points (95% CI, 5.2-10.8) P = 0.000. NeuroEPO plus treatment induced a statistically significant improvement in some of clinical secondary outcomes vs. placebo including CIBIC+, GDS, MoCA, NPI, and the brain perfusion.
Conclusions: Among participants with mild-moderate Alzheimer's disease clinical syndrome, neuroEPO plus improved the cognitive evaluation at 48 weeks, with a very good safety profile. Larger trials are warranted to determine the efficacy and safety of neuroEPO plus in Alzheimer's disease.
Trial registration: https://rpcec.sld.cu Identifier: RPCEC00000232.
Keywords: Alzheimer’s disease; NeuroEPO; Neuroprotective; Randomized controlled trial.
© 2023. The Author(s).
Conflict of interest statement
K. León, T. Crombet, T. Rodríguez and L. Pérez are employees of Center of Molecular Immunology, the institution that produces the Investigational New Drug (IND). T. Rodríguez is one of the authors of the patent “Human recombinant hyposialylated erythropoietin, methods of purification and therapeutic uses thereof” PCT US2022/0305084; no further authors have anything to disclose.
Figures

References
-
- U.S. Food and Drug Administration (FDA). FDA converts novel Alzheimer’s disease treatment to traditional approval. FDA News Release; 2021. https://www.fda.gov/news-events/press-announcements/fda-converts-novel-a.... Accessed 19 Jul 2023.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

