The traditional Chinese medicine Qiliqiangxin in heart failure with reduced ejection fraction: a randomized, double-blind, placebo-controlled trial
- PMID: 39095596
- PMCID: PMC11333273
- DOI: 10.1038/s41591-024-03169-2
The traditional Chinese medicine Qiliqiangxin in heart failure with reduced ejection fraction: a randomized, double-blind, placebo-controlled trial
Abstract
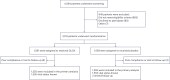
Previous findings have indicated the potential benefits of the Chinese traditional medicine Qiliqiangxin (QLQX) in heart failure. Here we performed a double-blind, randomized controlled trial to evaluate the efficacy and safety of QLQX in patients with heart failure and reduced ejection fraction (HFrEF). This multicenter trial, conducted in 133 hospitals in China, enrolled 3,110 patients with HFrEF with NT-proBNP levels of ≥450 pg ml-1 and left ventricular ejection fraction of ≤40%. Participants were randomized to receive either QLQX capsules or placebo (four capsules three times daily) alongside standard heart failure therapy. The trial met its primary outcome, which was a composite of hospitalization for heart failure and cardiovascular death: over a median follow-up of 18.3 months, the primary outcome occurred in 389 patients (25.02%) in the QLQX group and 467 patients (30.03%) in the placebo group (hazard ratio (HR), 0.78; 95% confidence interval (CI), 0.68-0.90; P < 0.001). In an analysis of secondary outcomes, the QLQX group showed reductions in both hospitalization for heart failure (15.63% versus 19.16%; HR, 0.76; 95% CI, 0.64-0.90; P = 0.002) and cardiovascular death (13.31% versus 15.95%; HR, 0.83; 95% CI, 0.68-0.996; P = 0.045) compared to the placebo group. All-cause mortality did not differ significantly between the two groups (HR, 0.84; 95% CI, 0.70-1.01; P = 0.058) and adverse events were also comparable between the groups. The results of this trial indicate that QLQX may improve clinical outcomes in patients with HFrEF when added to conventional therapy. ChiCTR registration: ChiCTR1900021929 .
© 2024. The Author(s).
Conflict of interest statement
X. Li and Z.J. are associate fellows at the State Key Laboratory for Innovation and Transformation of Luobing Theory. X. Li and H.Z. are associate fellows at the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine. X. Li reports receiving grant support (all grant support listed paid to the First Affiliated Hospital with Nanjing Medical University) from Novartis and the China Heart Failure Center and receiving lecture fees and consulting fees from AstraZeneca, Bayer, Novartis, Roche and Yiling. Z.J. reports affiliation with Hebei Yiling Hospital, a nonprofit medical institution, which is a completely independent legal entity from Shijiazhuang Yiling Pharmaceutical. Z.J. is the spouse of R.W., who holds shares and serves as a director of Shijiazhuang Yiling Pharmaceutical. Z.J. has fully disclosed these interests to the research committee and has developed an approved plan to manage any potential conflicts that may arise from such an arrangement and ensured the scientificity, objectivity and authority of the research results. H.Z. reports receiving lecture fees from AstraZeneca, Bayer, Novartis and Servier. I.C. reports receiving lecture fees from Haoyishu and Novartis. W.Y. reports receiving lecture fees from Bayer, Novartis, Sanofi and Yiling. Y.Z. reports receiving lecture fees from Novartis. S. Liao reports receiving lecture fees from Novartis. J.W. reports receiving lecture fees from AstraZeneca and Novartis. C.C. reports receiving lecture fees and consulting fees from AstraZeneca, Bayer and Pfizer. Q.R. reports receiving lecture fees and consulting fees from Bayer, Qilu pharmaceutical and China Medical System. No other potential competing interests relevant to this article are reported.
Figures




References
-
- DeVore, A. D. et al. Effect of a hospital and postdischarge quality improvement intervention on clinical outcomes and quality of care for patients with heart failure with reduced ejection fraction: the CONNECT-HF randomized clinical trial. JAMA326, 314–323 (2021). 10.1001/jama.2021.8844 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

