Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study
- PMID: 18852164
- PMCID: PMC2658827
- DOI: 10.1136/bmj.a1754
Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study
Abstract
Objective: To obtain large scale and generalisable data on the long term predictive value of cytology and human papillomavirus (HPV) testing for development of cervical intraepithelial neoplasia grade 3 or cancer (CIN3+).
Design: Multinational cohort study with joint database analysis.
Setting: Seven primary HPV screening studies in six European countries.
Participants: 24,295 women attending cervical screening enrolled into HPV screening trials who had at least one cervical cytology or histopathology examination during follow-up.
Main outcome measure: Long term cumulative incidence of CIN3+.
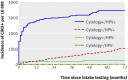
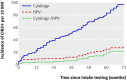
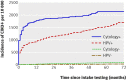
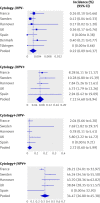
Results: The cumulative incidence rate of CIN3+ after six years was considerably lower among women negative for HPV at baseline (0.27%, 95% confidence interval 0.12% to 0.45%) than among women with negative results on cytology (0.97%, 0.53% to 1.34%)). By comparison, the cumulative incidence rate for women with negative cytology results at the most commonly recommended screening interval in Europe (three years) was 0.51% (0.23% to 0.77%). The cumulative incidence rate among women with negative cytology results who were positive for HPV increased continuously over time, reaching 10% at six years, whereas the rate among women with positive cytology results who were negative for HPV remained below 3%.
Conclusions: A consistently low six year cumulative incidence rate of CIN3+ among women negative for HPV suggests that cervical screening strategies in which women are screened for HPV every six years are safe and effective.
Conflict of interest statement
Competing interests: K-UP has received speaker’s honorarium from Digene and Roche and research grants from Digene; TI has received speaker’s honorarium from Digene; JC is on the speaker’s bureau of Digene and the advisory board for Roche and has received research grants from Roche; SK is on an advisory board for Merck and has received research grants from Merck and SPMSD; CM has received travel grants from Merck; SdeS has received travel grants from Digene and GSK and research grants from GSK and Merck/Sanofi Pasteur. The Department of Public Health of the Erasmus MC in Rotterdam, Netherlands, has received a research grant from GSK.
Ethical approval: All studies were approved by the ethical review boards in their respective countries.
Figures
References
-
- Bray F, Loos AH, McCarron P, Weiderpass E, Arbyn M, Moller H, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev 2005;14:677-86. - PubMed
-
- Bray F, Sankila R, Ferlay J, Parkin D. Estimates of cancer incidence and mortality in Europe in 1995. Eur J Cancer 2002;38:99-166. - PubMed
-
- Stoler MH, Schiffman M. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL triage study. JAMA 2001;285:1500-5. - PubMed
-
- Cuzick J, Clavel C, Petry KU, Meijer CJ, Hoyer H, Ratnam S, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006;119:1095-101. - PubMed
-
- Arbyn M, Sasieni P, Meijer C, Clavel C, Kolipoulos G, Dillner J. Chapter 9: Clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006;24(suppl 3):78-89. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
