A multinational study of thromboprophylaxis practice in critically ill children
- PMID: 24351371
- PMCID: PMC4293013
- DOI: 10.1097/CCM.0000000000000147
A multinational study of thromboprophylaxis practice in critically ill children
Abstract
Objectives: Although critically ill children are at increased risk for developing deep venous thrombosis, there are few pediatric studies establishing the prevalence of thrombosis or the efficacy of thromboprophylaxis. We tested the hypothesis that thromboprophylaxis is infrequently used in critically ill children even for those in whom it is indicated.
Design: Prospective multinational cross-sectional study over four study dates in 2012.
Setting: Fifty-nine PICUs in Australia, Canada, New Zealand, Portugal, Singapore, Spain, and the United States.
Patients: All patients less than 18 years old in the PICU during the study dates and times were included in the study, unless the patients were 1) boarding in the unit waiting for a bed outside the PICU or 2) receiving therapeutic anticoagulation.
Interventions: None.
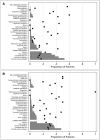
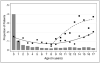
Measurements and main results: Of 2,484 children in the study, 2,159 (86.9%) had greater than or equal to 1 risk factor for thrombosis. Only 308 children (12.4%) were receiving pharmacologic thromboprophylaxis (e.g., aspirin, low-molecular-weight heparin, or unfractionated heparin). Of 430 children indicated to receive pharmacologic thromboprophylaxis based on consensus recommendations, only 149 (34.7%) were receiving it. Mechanical thromboprophylaxis was used in 156 of 655 children (23.8%) 8 years old or older, the youngest age for that device. Using nonlinear mixed effects model, presence of cyanotic congenital heart disease (odds ratio, 7.35; p < 0.001) and spinal cord injury (odds ratio, 8.85; p = 0.008) strongly predicted the use of pharmacologic and mechanical thromboprophylaxis, respectively.
Conclusions: Thromboprophylaxis is infrequently used in critically ill children. This is true even for children at high risk of thrombosis where consensus guidelines recommend pharmacologic thromboprophylaxis.
Conflict of interest statement
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures

Comment in
-
The landscape of thromboprophylaxis utilization in critically ill children: sparse and variable.Crit Care Med. 2014 May;42(5):1317-8. doi: 10.1097/CCM.0000000000000185. Crit Care Med. 2014. PMID: 24736356 No abstract available.
References
-
- Patel R, Cook DJ, Meade MO, et al. Burden of Illness in venous ThromboEmbolism in Critical care (BITEC) Study Investigators; Canadian Critical Care Trials Group. Burden of illness in venous thromboembolism in critical care: A multicenter observational study. J Crit Care. 2005;20:341–347. - PubMed
-
- Alhazzani W, Lim W, Jaeschke RZ, et al. Heparin thromboprophylaxis in medical-surgical critically ill patients: A systematic review and meta-analysis of randomized trials. Crit Care Med. 2013;41:2088–2098. - PubMed
-
- Higgerson RA, Lawson KA, Christie LM, et al. National Association of Children's Hospitals and Related Institution's Pediatric Intensive Care Unit FOCUS group. Incidence and risk factors associated with venous thrombotic events in pediatric intensive care unit patients. Pediatr Crit Care Med. 2011;12:628–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

