Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: results of a randomized, double-blind clinical trial
- PMID: 28705252
- PMCID: PMC5513031
- DOI: 10.1186/s12888-017-1412-1
Efficacy of prospective pharmacogenetic testing in the treatment of major depressive disorder: results of a randomized, double-blind clinical trial
Abstract
Background: A 12-week, double-blind, parallel, multi-center randomized controlled trial in 316 adult patients with major depressive disorder (MDD) was conducted to evaluate the effectiveness of pharmacogenetic (PGx) testing for drug therapy guidance.
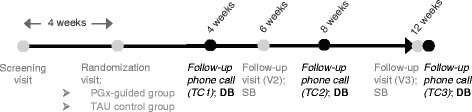
Methods: Patients with a CGI-S ≥ 4 and requiring antidepressant medication de novo or changes in their medication regime were recruited at 18 Spanish public hospitals, genotyped with a commercial PGx panel (Neuropharmagen®), and randomized to PGx-guided treatment (n = 155) or treatment as usual (TAU, control group, n = 161), using a computer-generated random list that locked or unlocked psychiatrist access to the results of the PGx panel depending on group allocation. The primary endpoint was the proportion of patients achieving a sustained response (Patient Global Impression of Improvement, PGI-I ≤ 2) within the 12-week follow-up. Patients and interviewers collecting the PGI-I ratings were blinded to group allocation. Between-group differences were evaluated using χ2-test or t-test, as per data type.
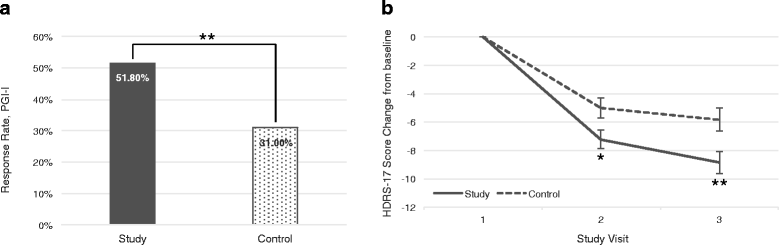
Results: Two hundred eighty patients were available for analysis at the end of the 12-week follow-up (PGx n = 136, TAU n = 144). A difference in sustained response within the study period (primary outcome) was not observed (38.5% vs 34.4%, p = 0.4735; OR = 1.19 [95%CI 0.74-1.92]), but the PGx-guided treatment group had a higher responder rate compared to TAU at 12 weeks (47.8% vs 36.1%, p = 0.0476; OR = 1.62 [95%CI 1.00-2.61]), and this difference increased after removing subjects in the PGx-guided group when clinicians explicitly reported not to follow the test recommendations (51.3% vs 36.1%, p = 0.0135; OR = 1.86 [95%CI 1.13-3.05]). Effects were more consistent in patients with 1-3 failed drug trials. In subjects reporting side effects burden at baseline, odds of achieving a better tolerability (Frequency, Intensity and Burden of Side Effects Rating Burden subscore ≤2) were higher in the PGx-guided group than in controls at 6 weeks and maintained at 12 weeks (68.5% vs 51.4%, p = 0.0260; OR = 2.06 [95%CI 1.09-3.89]).
Conclusions: PGx-guided treatment resulted in significant improvement of MDD patient's response at 12 weeks, dependent on the number of previously failed medication trials, but not on sustained response during the study period. Burden of side effects was also significantly reduced.
Trial registration: European Clinical Trials Database 2013-002228-18 , registration date September 16, 2013; ClinicalTrials.gov NCT02529462 , retrospectively registered: August 19, 2015.
Keywords: Antidepressant response; Depression; Pharmacogenetics; Precision medicine; Randomized clinical trial.
Conflict of interest statement
Ethics approval and consent to participate
This prospective, multicenter, randomized, double-blind, naturalistic clinical study was approved by the Institutional Review Board (IRB) of Hospital Clínic de Barcelona (Spain), acting as a centralized reference IRB, as well as the IRB of each participating hospital (see ‘List of Ethic Committees’ in the Additional file 2). The study was conducted in compliance with Good Clinical Practice requirements and the Declaration of Helsinki. Written informed consent was obtained from all participants before enrolment.
Consent for publication
Not applicable.
Competing interests
VP has received grants and served as consultant, advisor or CME speaker for AB-Biotics, AstraZeneca, Bristol-Myers Squibb, Glaxo-Smith-Kline, Pfizer, Janssen, Lundbeck, Medtronic, Otsuka, and the Spanish Ministry of Economy and Competitiveness (Centro de Investigación Biomédica en Red de Salud Mental, CIBERSAM). AS and JE are employees of and minor stock shareholders in AB-Biotics. MT is employee of AB-Biotics. JSR has received grants and served as consultant, advisor or CME speaker for Lundbeck, Janssen and Otsuka. JB1 has received grants and served as consultant, advisor or CME speaker for AB-Biotics, Adamed, Almirall, AstraZeneca, Bristol-Myers Squibb, Ferrer, Glaxo-Smith-Kline, Hoffman-La Roche, Janssen-Cilag, Eli Lilly, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Sanofi-Aventis, Servier, Schering-Plough and Shire, and research funding from the Spanish Ministry of Economy and Competitiveness (CIBERSAM, Instituto de Salud Carlos III, ISCIII), Spanish Ministry of Health, Social Services and Equality (Plan Nacional sobre Drogas) and the European Union’s 7th Framework Program (EU FP7). EB has received grants from Janssen, Otsuka and Servier. EV has received grants and served as consultant, advisor or CME speaker for AB-Biotics, Actavis, Allergan, AstraZeneca, Bial, Bristol-Myers Squibb, Dainippon Sumitomo Pharma, Elan, Eli Lilly, Farmindustria, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Solvay, Sunovion, Takeda, Telefonica, the Brain & Behavior Research Foundation (NARSAD), CIBERSAM, the EU FP7 (ENBREC), and the Stanley Medical Research Institute. JMO has received grants and served as consultant, advisor or CME speaker for AstraZeneca, Bristol-Myers Squibb, Glaxo-Smith-Kline, Pfizer, Janssen, Lundbeck, Otsuka, Eli Lilly, Novartis, and Sanofi-Aventis. RRJ has received grants and served as consultant, advisor or CME speaker for ISCIII, Fondo de Investigación Sanitaria (FIS), CIBERSAM, Madrid Regional Government (S2010/BMD-2422 AGES), Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Ferrer, Juste. JMV has received grants and served as consultant, advisor or CME speaker for Janssen-Cilag, Pfizer, Bristol-Myers Squibb, Otsuka, Glaxo-Smith-Kline, AstraZeneca, Sanofi-Aventis, Novartis, Lundbeck and Ferrer. JG has received grants and served as consultant, advisor or CME speaker for Janssen-Cilag, Lundbeck, Pfizer and BioClever 2005. JCC has received grants and served as consultant for Lundbeck/Otsuka. PAS has received grants or served as consultant for Adamed, AstraZeneca, Brainpharma, Bristol-Myers Squibb, Esteve, Ferrer inCode, Glaxo-Smith-Kline, Janssen-Cilag, Eli Lilly, Lundbeck, Otsuka, Pfizer, Rovi, Servier, the Spanish Ministry of Health, Social Services and Equality (Plan Nacional sobre Drogas), CIBERSAM, and the EU FP7. AI has served as speaker or advisor for Bristol-Myers Squibb, Ferrer, Lundbeck, Otsuka and Servier. JDA is funded by the Instituto de Salud Carlos III through a ‘Juan Rodés’ research fellowship (JR14/00011) and has received lecture honoraria from Pfizer, Glaxo-Smith-Kine and Lundbeck. JMM1 has received grants and served as consultant, advisor or CME speaker for AB-Biotics, Ferrer, Glaxo-Smith-Kline, Janssen, Lundbeck, Medtronic, Otsuka, and CIBERSAM. EA1 has received grants and served as consultant, advisor or CME speaker for AB-Biotics, Eli Lilly, Lundbeck, Pfizer, Sanofi-Aventis, Bristol-Myers Squibb, Servier and Otsuka. FMC has served as consultant for Janssen-Cilag. JQ has received grants, served as consultant or CME speaker for Janssen, Otsuka, Shire, Mutua Madrileña & Grunethal. DJP has received grants and served as consultant for Lundbeck/Otsuka. MG has received grants and served as consultant, advisor or CME speaker for Ferrer, Janssen, Lundbeck and the Spanish Ministry of Economy and Competitiveness (ISCIII). VS has received grants and served as consultant or CME speaker for Servier, Rovi, Lundbeck, Pfizer and the Spanish Ministry of Science and Innovation. CDA has received grants and served as consultant, advisor or CME speaker for Lundbeck, Rovi, Eli Lilly and Pfizer. JMM2 has received grants and served as CME speaker for Janssen-Cilag, Pfizer, Otsuka, AstraZeneca, Sanofi-Aventis, Lundbeck and Ferrer. FM has received grants and served as consultant, advisor or CME speaker for AstraZeneca, Ferrer, Janssen, Glaxo-Smith-Kline, Pfizer, Otsuka and Lundbeck. PMH has received grants and served as consultant, advisor or CME speaker for Janssen-Cilag, Lundbeck, Otsuka, Boehringer Ingelheim. EA2 has received grants and served as CME speaker for Janssen-Cilag and Otsuka. MPGP has received grants or served as consultant for Lundbeck/Otsuka, ISCIII, CIBERSAM, Janssen-Cilag, Eli Lilly, Lundbeck, Otsuka, Pfizer, Servier, Roche and Rovi. MB has received grants and served as consultant, advisor or CME speaker for AB-Biotics, Adamed, Almirall, Amgen, Boehringer, Eli Lilly, Ferrer, Forum Pharmaceuticals, Gedeon Richter, Hersill, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Roche, Servier and has obtained research funding from the Ministry of Education, Culture and Sports, ISCIII, CIBERSAM, the Government of Catalonia, Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2014SGR441), European Group for Research In Schizophrenia (EGRIS) Foundation, and the EU FP7. MP and NR are employees of AB-Biotics. MAB is employee of and stock shareholder in AB-Biotics. All other authors declare no conflicts of interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden Of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

