Bilateral remote ischemic conditioning in children: A two-center, double-blind, randomized controlled trial in young children undergoing cardiac surgery
- PMID: 38690427
- PMCID: PMC11056492
- DOI: 10.1016/j.xjon.2024.02.018
Bilateral remote ischemic conditioning in children: A two-center, double-blind, randomized controlled trial in young children undergoing cardiac surgery
Abstract
Objective: The study objective was to determine whether adequately delivered bilateral remote ischemic preconditioning is cardioprotective in young children undergoing surgery for 2 common congenital heart defects with or without cyanosis.
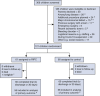
Methods: We performed a prospective, double-blind, randomized controlled trial at 2 centers in the United Kingdom. Children aged 3 to 36 months undergoing tetralogy of Fallot repair or ventricular septal defect closure were randomized 1:1 to receive bilateral preconditioning or sham intervention. Participants were followed up until hospital discharge or 30 days. The primary outcome was area under the curve for high-sensitivity troponin-T in the first 24 hours after surgery, analyzed by intention-to-treat. Right atrial biopsies were obtained in selected participants.
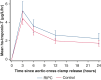
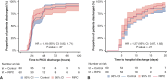
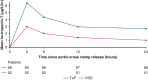
Results: Between October 2016 and December 2020, 120 eligible children were randomized to receive bilateral preconditioning (n = 60) or sham intervention (n = 60). The primary outcome, area under the curve for high-sensitivity troponin-T, was higher in the preconditioning group (mean: 70.0 ± 50.9 μg/L/h, n = 56) than in controls (mean: 55.6 ± 30.1 μg/L/h, n = 58) (mean difference, 13.2 μg/L/h; 95% CI, 0.5-25.8; P = .04). Subgroup analyses did not show a differential treatment effect by oxygen saturations (pinteraction = .25), but there was evidence of a differential effect by underlying defect (pinteraction = .04). Secondary outcomes and myocardial metabolism, quantified in atrial biopsies, were not different between randomized groups.
Conclusions: Bilateral remote ischemic preconditioning does not attenuate myocardial injury in children undergoing surgical repair for congenital heart defects, and there was evidence of potential harm in unstented tetralogy of Fallot. The routine use of remote ischemic preconditioning cannot be recommended for myocardial protection during pediatric cardiac surgery.
Keywords: clinical trial; cyanosis; myocardial protection; pediatric cardiac surgery; remote ischemic preconditioning; tetralogy of Fallot.
© 2024 The Author(s).
Conflict of interest statement
The authors reported no conflicts of interest. The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Figures




References
-
- Mildh L.H., Pettilä V., Sairanen H.I., Rautiainen P.H. Cardiac troponin T levels for risk stratification in pediatric open heart surgery. Ann Thorac Surg. 2006;82:1643–1649. - PubMed
-
- Ma M., Gauvreau K., Allan C.K., Mayer J.E., Jr., Jenkins K.J. Causes of death after congenital heart surgery. Ann Thorac Surg. 2007;83:1438–1445. - PubMed
-
- Najm H.K., Wallen W.J., Belanger M.P., et al. Does the degree of cyanosis affect myocardial adenosine triphosphate levels and function in children undergoing surgical procedures for congenital heart disease? J Thorac Cardiovasc Surg. 2000;119:515–524. - PubMed
-
- Imura H., Caputo M., Parry A., Pawade A., Angelini G.D., Suleiman M.S. Age-dependent and hypoxia-related differences in myocardial protection during paediatric open-heart surgery. Circulation. 2001;103:1551–1556. - PubMed
E-References
-
- Norozi K., Beck C., Osthaus W.A., Wille I., Wessel A., Bertram H. Electrical velocimetry for measuring cardiac output in children with congenital heart disease. Br J Anaesth. 2008;100:88–94. - PubMed
-
- Schubert S., Schmitz T., Weiss M., et al. Continuous, non-invasive techniques to determine cardiac output in children after cardiac surgery: evaluation of transoesophageal Doppler and electric velocimetry. J Clin Monit Comput. 2008;22:299–307. - PubMed
-
- Southam A.D., Pursell H., Frigerio G., Jankevics A., Weber R.J.M., Dunn W.B. Characterization of monophasic solvent-based tissue extractions for the detection of polar metabolites and lipids applying ultrahigh-performance liquid chromatography-mass spectrometry clinical metabolic phenotyping assays. J Proteome Res. 2021;20:831–840. - PubMed
-
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57:289–300.
Publication types
LinkOut - more resources
Full Text Sources

