SCAview: an Intuitive Visual Approach to the Integrative Analysis of Clinical Data in Spinocerebellar Ataxias
- PMID: 37002505
- PMCID: PMC10544694
- DOI: 10.1007/s12311-023-01546-0
SCAview: an Intuitive Visual Approach to the Integrative Analysis of Clinical Data in Spinocerebellar Ataxias
Abstract
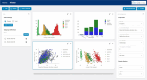
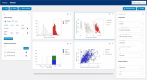
With SCAview, we present a prompt and comprehensive tool that enables scientists to browse large datasets of the most common spinocerebellar ataxias intuitively and without technical effort. Basic concept is a visualization of data, with a graphical handling and filtering to select and define subgroups and their comparison. Several plot types to visualize all data points resulting from the selected attributes are provided. The underlying synthetic cohort is based on clinical data from five different European and US longitudinal multicenter cohorts in spinocerebellar ataxia type 1, 2, 3, and 6 (SCA1, 2, 3, and 6) comprising > 1400 patients with overall > 5500 visits. First, we developed a common data model to integrate the clinical, demographic, and characterizing data of each source cohort. Second, the available datasets from each cohort were mapped onto the data model. Third, we created a synthetic cohort based on the cleaned dataset. With SCAview, we demonstrate the feasibility of mapping cohort data from different sources onto a common data model. The resulting browser-based visualization tool with a thoroughly graphical handling of the data offers researchers the unique possibility to visualize relationships and distributions of clinical data, to define subgroups and to further investigate them without any technical effort. Access to SCAview can be requested via the Ataxia Global Initiative and is free of charge.
Keywords: Observational studies; Spinocerebellar ataxia (SCA); Visualization.
© 2023. The Author(s).
Conflict of interest statement
Dr. Klockgether is receiving research support from the Bundesministerium für Bildung und Forschung (BMBF), the National Institutes of Health (NIH) and Servier. Within the last 24 months, he has received consulting fees from Biogen, UCB, and Vico Therapeutics. Dr. Ashizawa receive NIH grants U01NS104326, R01NS115002, and R01NS124065. Dr. Ashizawa also received grants from the National Ataxia Foundation (NAF), and participated in Biohaven 201 and 206 clinical trials. Dr. Kuo is funded by the National Institutes of Health. Dr. Kuo serves as scientific advisor for Praxis Precision Medicine, Sage Therapeutics, Neurocrine Biosciences, and Reata Pharmaceuticals. Dr. Faber receives funding as a fellow of the Hertie Network of Excellence in Neuroscience and from the National Ataxia Foundation (NAF). All other authors have no competing interests.
Figures