Intramuscular depot medroxyprogesterone acetate accentuates bone loss associated with tenofovir disoproxil fumarate-containing antiretroviral therapy initiation in young women living with HIV (the BONE: CARE study): a prospective cohort study in Uganda
- PMID: 35427526
- PMCID: PMC9449816
- DOI: 10.1016/S2214-109X(22)00080-8
Intramuscular depot medroxyprogesterone acetate accentuates bone loss associated with tenofovir disoproxil fumarate-containing antiretroviral therapy initiation in young women living with HIV (the BONE: CARE study): a prospective cohort study in Uganda
Abstract
Background: Tenofovir disoproxil fumarate (TDF) and intramuscular depot medroxyprogesterone acetate (DMPA-IM) are independently associated with reduced bone mineral density (BMD). We aimed to assess the combined effects of DMPA-IM use and TDF initiation on BMD in young adult women living with HIV over two years, compared with age-matched people without HIV.
Methods: Th BONE: CARE study was a prospective cohort study that recruited women aged 18-35 years from 11 HIV care and general health facilities in Kampala, Uganda. The participants were classified into four groups on the basis of their combination of HIV status, TDF use, and DMPA-IM use, as follows: women living with HIV initiating TDF-containing antiretroviral therapy (ART) with DMPA-IM (HIV positive, DMPA positive, and TDF positive); women living with HIV using DMPA-IM but not eligible for ART as per local guidelines at the time of enrolment into the study (HIV positive, DMPA positive, and TDF negative); women living with HIV initiating TDF-containing ART without DMPA-IM (HIV positive, DMPA negative, and TDF positive); and controls without HIV using non-hormonal contraceptives (HIV negative, DMPA negative, and TDF negative). BMD of the lumbar spine, total hip, and femoral neck were measured using semiannual dual-energy x-ray absorptiometry at enrolment and at intervals every 6 months thereafter. We assessed percentage change in mean BMD.
Findings: Between March 30, 2016, and Oct 19, 2017, we enrolled 265 women living with HIV initiating ART (159 DMPA-IM users and 106 non-hormonal contraceptive users), 187 women living with HIV using DMPA-IM but not ART, and 69 controls without HIV. Mean age was 26·1 years (SD 4·2). BMD declined significantly from baseline in women living with HIV on TDF with versus without DMPA-IM at the lumbar spine (-3·406% [95% CI -3·969 to -2·844] vs -1·111% [-1·929 to -0·293]; p<0·0001), total hip (-3·856% [-4·449 to -3·264] vs -1·714% [-2·479 to -0·949]; p=0·0002), and femoral neck (-4·422% [-5·078 to -3·766] vs -1·999% [-3·022 to -0·976]; p=0·0002), increased in controls at the lumbar spine (1·5% change), and remained unchanged at total hip and femoral neck (-0·1% change). Concurrent use of TDF and DMPA-IM resulted in significantly greater BMD decline (p<0·0001) than TDF alone (lumbar spine -2·677% [95% CI -3·743 to -1·611]; p<0·0001; total hip -2·518% [-3·575 to -1·461]; p<0·0001; and femoral neck -2·907 [-4·132 to -1·683]; p<0·0001) or than controls (lumbar spine -4·970% [-6·391 to -3·549]; p<0·0001; total hip -4·151% [-5·579 to -2·724]; p<0.0001; and femoral neck -4·773% [-6·424 to -3·122]; p<0·0001) INTERPRETATION: Concomitant DMPA-IM use resulted in a doubling of BMD loss in women living with HIV initiating TDF-containing ART. Identification of safer contraceptive and bone-sparing ART options should be prioritised for optimal care of women living with HIV.
Funding: National Institute of Allergy and Infectious Diseases of the US National Institutes of Health.
Copyright © 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests TTB has received consulting fees from Merck, ViiV Heathcare, Gilead Sciences, Janssen, and Theratecnologies. FKM has received money to her institution from Gilead Sciences. All other authors declare no competing interests.
Figures
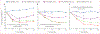
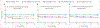
Comment in
-
Challenges to current and future bone health in young women living with HIV.Lancet Glob Health. 2022 May;10(5):e598-e599. doi: 10.1016/S2214-109X(22)00178-4. Lancet Glob Health. 2022. PMID: 35427508 No abstract available.
References
-
- UNAIDS. Fact sheet World Aids Day 2020. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_e... (accessed April 20, 2021).
-
- Egger M, Johnson LF. Estimating trends in life expectancy in HIV-positive individuals. Lancet Glob Health 2015; 3: e122–23. - PubMed
-
- Premaor MO, Compston JE. People living with HIV and fracture risk. Osteoporos Int 2020; 31: 1633–44. - PubMed
-
- Borges ÁH, Hoy J, Florence E, et al. Antiretrovirals, fractures, and osteonecrosis in a large international HIV cohort. Clin Infect Dis 2017; 64: 1413–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

