Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial
- PMID: 21072246
- PMCID: PMC2970549
- DOI: 10.1371/journal.pmed.1000362
Efficacy of oseltamivir-zanamivir combination compared to each monotherapy for seasonal influenza: a randomized placebo-controlled trial
Erratum in
- PLoS Med. 2010;7(12) doi: 10.1371/annotation/ca448e7c-fbbc-43e9-9981-108c9bfa8bce
Abstract
Background: Neuraminidase inhibitors are thought to be efficacious in reducing the time to alleviation of symptoms in outpatients with seasonal influenza. The objective of this study was to compare the short-term virological efficacy of oseltamivir-zanamivir combination versus each monotherapy plus placebo.
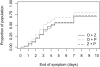
Methods and findings: We conducted a randomized placebo-controlled trial with 145 general practitioners throughout France during the 2008-2009 seasonal influenza epidemic. Patients, general practitioners, and outcome assessors were all blinded to treatment assignment. Adult outpatients presenting influenza-like illness for less than 36 hours and a positive influenza A rapid test diagnosis were randomized to oseltamivir 75 mg orally twice daily plus zanamivir 10 mg by inhalation twice daily (OZ), oseltamivir plus inhaled placebo (O), or zanamivir plus oral placebo (Z). Treatment efficacy was assessed virologically according to the proportion of patients with nasal influenza reverse transcription (RT)-PCR below 200 copies genome equivalent (cgeq)/µl at day 2 (primary outcome), and clinically to the time to alleviation of symptoms until day 14. Overall 541 patients (of the 900 planned) were included (OZ, =192; O, n=176; Z, n=173), 49% male, mean age 39 years. In the intention-to-treat analysis conducted in the 447 patients with RT-PCR-confirmed influenza A, 46%, 59%, and 34% in OZ (n=157), O (n=141), and Z (n=149) arms had RT-PCR<200 cgeq/µl (-13.0%, 95% confidence interval [CI] -23.1 to -2.9, p=0.025; +12.3%, 95% CI 2.39-22.2, p=0.028 for OZ/O and OZ/Z comparisons). Mean day 0 to day 2 viral load decrease was 2.14, 2.49, and 1.68 log(10) cgeq/µl (p=0.060, p=0.016 for OZ/O and OZ/Z). Median time to alleviation of symptoms was 4.0, 3.0, and 4.0 days (+1.0, 95% CI 0.0-4.0, p=0.018; +0.0, 95% CI -3.0 to 3.0, p=0.960 for OZ/O and OZ/Z). Four severe adverse events were observed. Nausea and/or vomiting tended to be more frequent in the combination arm (OZ, n=13; O, n=4; and Z, n=5 patients, respectively).
Conclusions: In adults with seasonal influenza A mainly H3N2 virus infection, the oseltamivir-zanamivir combination appeared less effective than oseltamivir monotherapy, and not significantly more effective than zanamivir monotherapy. Despite the theoretical potential for the reduction of the emergence of antiviral resistance, the lower effectiveness of this combination calls for caution in its use in clinical practice.
Trial registration: www.ClinicalTrials.govNCT00799760.
Conflict of interest statement
XD has had a conference invitation from GSK and lecture fees from Roche and Gilead. AM has membership in the ministry of health advisory board on influenza; involvement in some epidemiological studies partially or fully granted by Roche and GSK, and travel grants from Roche for participation in scientific meetings. SVDW has had a conference invitation from GSK; research grant from GSK on unrelated subject; joined patent from institution with GSK on unrelated subject; travel grants for meetings from GSK; contribution to clinical trial financed by Roche; member of the advisory committee on influenza of the French ministry of health; is a member of ESWI; is a member of the scientific committee of the GEIG; and is vice-president of the GROG network. FM received fees from Roche, preclinical pharmacokinetic department, for a course on MONOLIX in December 2008. BL has had paid consultancy and board membership (Roche, GSK, Novartis, BioCryst, MedImmune), has had research grants from Roche and Sanofi-Pasteur, and had received travel grants and honoraria for speaking or participation at meetings (Roche, Sanofi-Pasteur).
Figures
References
-
- Burch J, Corbett M, Stock C, Nicholson K, Elliot AJ, et al. Prescription of anti-influenza drugs for healthy adults: a systematic review and meta-analysis. Lancet Infect Dis. 2009;9:537–545. - PubMed
-
- Hayden FG, Osterhaus AD, Treanor JJ, Fleming DM, Aoki FY, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections. GG167 Influenza Study Group. N Engl J Med. 1997;337:874–880. - PubMed
-
- Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–1850. - PubMed
-
- Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–1024. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

