Intrathecal Gene Therapy for Giant Axonal Neuropathy
- PMID: 38507752
- PMCID: PMC11973737
- DOI: 10.1056/NEJMoa2307952
Intrathecal Gene Therapy for Giant Axonal Neuropathy
Abstract
Background: Giant axonal neuropathy is a rare, autosomal recessive, pediatric, polysymptomatic, neurodegenerative disorder caused by biallelic loss-of-function variants in GAN, the gene encoding gigaxonin.
Methods: We conducted an intrathecal dose-escalation study of scAAV9/JeT-GAN (a self-complementary adeno-associated virus-based gene therapy containing the GAN transgene) in children with giant axonal neuropathy. Safety was the primary end point. The key secondary clinical end point was at least a 95% posterior probability of slowing the rate of change (i.e., slope) in the 32-item Motor Function Measure total percent score at 1 year after treatment, as compared with the pretreatment slope.
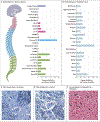
Results: One of four intrathecal doses of scAAV9/JeT-GAN was administered to 14 participants - 3.5×1013 total vector genomes (vg) (in 2 participants), 1.2×1014 vg (in 4), 1.8×1014 vg (in 5), and 3.5×1014 vg (in 3). During a median observation period of 68.7 months (range, 8.6 to 90.5), of 48 serious adverse events that had occurred, 1 (fever) was possibly related to treatment; 129 of 682 adverse events were possibly related to treatment. The mean pretreatment slope in the total cohort was -7.17 percentage points per year (95% credible interval, -8.36 to -5.97). At 1 year after treatment, posterior mean changes in slope were -0.54 percentage points (95% credible interval, -7.48 to 6.28) with the 3.5×1013-vg dose, 3.23 percentage points (95% credible interval, -1.27 to 7.65) with the 1.2×1014-vg dose, 5.32 percentage points (95% credible interval, 1.07 to 9.57) with the 1.8×1014-vg dose, and 3.43 percentage points (95% credible interval, -1.89 to 8.82) with the 3.5×1014-vg dose. The corresponding posterior probabilities for slowing the slope were 44% (95% credible interval, 43 to 44); 92% (95% credible interval, 92 to 93); 99% (95% credible interval, 99 to 99), which was above the efficacy threshold; and 90% (95% credible interval, 89 to 90). Between 6 and 24 months after gene transfer, sensory-nerve action potential amplitudes increased, stopped declining, or became recordable after being absent in 6 participants but remained absent in 8.
Conclusions: Intrathecal gene transfer with scAAV9/JeT-GAN for giant axonal neuropathy was associated with adverse events and resulted in a possible benefit in motor function scores and other measures at some vector doses over a year. Further studies are warranted to determine the safety and efficacy of intrathecal AAV-mediated gene therapy in this disorder. (Funded by the National Institute of Neurological Disorders and Stroke and others; ClinicalTrials.gov number, NCT02362438.).
Copyright © 2024 Massachusetts Medical Society.
Figures

References
-
- Opal P GAN-related neurodegeneration. In: Adam MP, ed. GeneReviews. Seattle: University of Washington, 2021. (https://www.ncbi.nlm.nih.gov/books/NBK1136/).
-
- Bomont P, Cavalier L, Blondeau F, et al. The gene encoding gigaxonin, a new member of the cytoskeletal BTB/kelch repeat family, is mutated in giant axonal neuropathy. Nat Genet 2000;26:370–4. - PubMed
-
- Allen E, Ding J, Wang W, et al. Gigaxonin-controlled degradation of MAP1B light chain is critical to neuronal survival. Nature 2005;438:224–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
