Comparison of dual therapies for hypertension treatment in India: a randomized clinical trial
- PMID: 40715816
- PMCID: PMC12443627
- DOI: 10.1038/s41591-025-03854-w
Comparison of dual therapies for hypertension treatment in India: a randomized clinical trial
Abstract
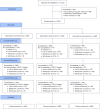
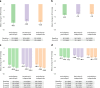
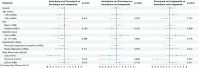
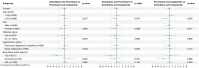
Evidence is lacking for guiding optimal combination hypertension therapy in South Asian patients. Here we investigated the blood pressure (BP)-lowering efficacy and safety of three commonly recommended antihypertensive dual combinations in a multicenter, single-blinded trial conducted in India. We randomized Indians aged 30-79 years (mean age, 52 years) with mean sitting systolic blood pressure (SBP) of 150-179 mmHg on no treatment or an SBP of 140-159 mmHg on monotherapy 1:1:1 to a single-pill combination of amlodipine-perindopril, perindopril-indapamide or amlodipine-indapamide. The primary outcome was the mean change in 24-hour ambulatory SBP at 6 months. Of the 1,981 participants (42% females) enrolled in the trial, 1,637 completed a 24-hour ambulatory BP measurement. All three drug combinations produced similar large reductions in the primary outcome, namely ambulatory (~14/8 mmHg) and office (~30/14 mmHg) BPs after 6 months, such that hypertension control rates (sitting BP < 140/90 mmHg) were achieved in approximately 70% of participants in all three groups. Furthermore, no significant differences in secondary outcomes, such as mean day and night ambulatory and office BPs and hypertension control rates, were observed among the study groups. Thus, in Indian patients, amlodipine-perindopril, perindopril-indapamide and amlodipine-indapamide were all equally well tolerated and equally highly effective in reducing 24-hour ambulatory and office BPs (ClinicalTrials.gov registration: NCT05683301 ).
© 2025. The Author(s).
Conflict of interest statement
Competing interests: N.P. has received financial support from several pharmaceutical companies that manufacture BP-lowering agents. N.P. has received consultancy fees (Servier and Aktiia), funding for research projects and staff (Servier and Pfizer) and fees for arranging and speaking at educational meetings (AstraZeneca, Lri Therapharma, Napi, Servier, Sanofi, Eva Pharma, Pfizer, Emcure India, Dr. Reddy’s Laboratories and Zydus). He holds no stocks or shares in any such company. D.P.’s institution, the Centre for Chronic Disease Control, has received research grants from Sun Pharmaceuticals, Lupin Limited and Intas Pharmaceuticals in India for capacity building of primary care physicians in cardiovascular diseases. The other authors declare no competing interests.
Figures
References
-
- World Health Organization. Global report on hypertension: the race against a silent killer. WHOhttps://www.who.int/publications/i/item/9789240081062 (2023).
-
- Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension71, 1269–1324 (2018). - PubMed
-
- Unger, T. et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension75, 1334–1357 (2020). - PubMed
-
- McEvoy, J. W. et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J.45, 3912–4018 (2024). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

