Treatment for Mild Chronic Hypertension during Pregnancy
- PMID: 35363951
- PMCID: PMC9575330
- DOI: 10.1056/NEJMoa2201295
Treatment for Mild Chronic Hypertension during Pregnancy
Abstract
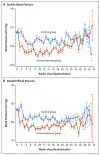
Background: The benefits and safety of the treatment of mild chronic hypertension (blood pressure, <160/100 mm Hg) during pregnancy are uncertain. Data are needed on whether a strategy of targeting a blood pressure of less than 140/90 mm Hg reduces the incidence of adverse pregnancy outcomes without compromising fetal growth.
Methods: In this open-label, multicenter, randomized trial, we assigned pregnant women with mild chronic hypertension and singleton fetuses at a gestational age of less than 23 weeks to receive antihypertensive medications recommended for use in pregnancy (active-treatment group) or to receive no such treatment unless severe hypertension (systolic pressure, ≥160 mm Hg; or diastolic pressure, ≥105 mm Hg) developed (control group). The primary outcome was a composite of preeclampsia with severe features, medically indicated preterm birth at less than 35 weeks' gestation, placental abruption, or fetal or neonatal death. The safety outcome was small-for-gestational-age birth weight below the 10th percentile for gestational age. Secondary outcomes included composites of serious neonatal or maternal complications, preeclampsia, and preterm birth.
Results: A total of 2408 women were enrolled in the trial. The incidence of a primary-outcome event was lower in the active-treatment group than in the control group (30.2% vs. 37.0%), for an adjusted risk ratio of 0.82 (95% confidence interval [CI], 0.74 to 0.92; P<0.001). The percentage of small-for-gestational-age birth weights below the 10th percentile was 11.2% in the active-treatment group and 10.4% in the control group (adjusted risk ratio, 1.04; 95% CI, 0.82 to 1.31; P = 0.76). The incidence of serious maternal complications was 2.1% and 2.8%, respectively (risk ratio, 0.75; 95% CI, 0.45 to 1.26), and the incidence of severe neonatal complications was 2.0% and 2.6% (risk ratio, 0.77; 95% CI, 0.45 to 1.30). The incidence of any preeclampsia in the two groups was 24.4% and 31.1%, respectively (risk ratio, 0.79; 95% CI, 0.69 to 0.89), and the incidence of preterm birth was 27.5% and 31.4% (risk ratio, 0.87; 95% CI, 0.77 to 0.99).
Conclusions: In pregnant women with mild chronic hypertension, a strategy of targeting a blood pressure of less than 140/90 mm Hg was associated with better pregnancy outcomes than a strategy of reserving treatment only for severe hypertension, with no increase in the risk of small-for-gestational-age birth weight. (Funded by the National Heart, Lung, and Blood Institute; CHAP ClinicalTrials.gov number, NCT02299414.).
Copyright © 2022 Massachusetts Medical Society.
Figures

Comment in
-
Treating Hypertension in Pregnancy.N Engl J Med. 2022 May 12;386(19):1846-1847. doi: 10.1056/NEJMe2203388. Epub 2022 Apr 2. N Engl J Med. 2022. PMID: 35363952 No abstract available.
-
Treatment of mild chronic hypertension in pregnancy reduces pre-eclampsia risk.Nat Rev Cardiol. 2022 Jun;19(6):350. doi: 10.1038/s41569-022-00712-x. Nat Rev Cardiol. 2022. PMID: 35444298 No abstract available.
-
Treatment for Mild Chronic Hypertension during Pregnancy.N Engl J Med. 2022 Aug 18;387(7):663-664. doi: 10.1056/NEJMc2207889. N Engl J Med. 2022. PMID: 36070721 No abstract available.
-
Treatment for Mild Chronic Hypertension during Pregnancy.N Engl J Med. 2022 Aug 18;387(7):664. doi: 10.1056/NEJMc2207889. N Engl J Med. 2022. PMID: 36070722 No abstract available.
References
-
- Ananth CV, Duzyj CM, Yadava S, Schwebel M, Tita ATN, Joseph KS. Changes in the prevalence of chronic hypertension in pregnancy, United States, 1970 to 2010. Hypertension 2019;74:1089–95. - PubMed
-
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy: report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. - PubMed
-
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins — Obstetrics. ACOG practice bulletin no. 203: chronic hypertension in pregnancy. Obstet Gynecol 2019;133(1):e26–e50. - PubMed
-
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
