Study protocol for the PICASSO trial: A randomized placebo-controlled trial to investigate the efficacy and safety of intraarticular steroid injections and an occupational therapy intervention in painful inflammatory carpometacarpal-1 osteoarthritis
- PMID: 39669005
- PMCID: PMC11636302
- DOI: 10.1016/j.ocarto.2024.100542
Study protocol for the PICASSO trial: A randomized placebo-controlled trial to investigate the efficacy and safety of intraarticular steroid injections and an occupational therapy intervention in painful inflammatory carpometacarpal-1 osteoarthritis
Abstract
Objective: Our primary objectives are to assess whether intraarticular corticosteroid injections are superior to saline injections with regards to thumb base pain after 4 weeks, and to compare the efficacy of steroid injections, saline injections, and an occupational therapy intervention on thumb base pain after 12 weeks in people with painful inflammatory osteoarthritis (OA) of the first carpometacarpal (CMC-1) joint.
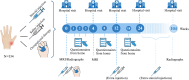
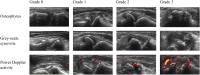
Design: In this three-armed, double-blind, randomized multicenter trial, 354 participants with painful inflammatory CMC-1 OA from six Norwegian hospitals are recruited. Participants are randomized 1:1:1 to intraarticular steroid or saline injections in the CMC-1 joint or a multimodal occupational therapy intervention. The primary outcomes are thumb base pain measured on a numeric rating scale (NRS, range: 0-10) after 4 weeks and 12 weeks. Key secondary outcomes include synovitis by Magnetic Resonance Imaging (MRI) after 4 weeks and hand function by the Measure of Activity Performance of the Hand (MAP-Hand) questionnaire after 12 and 24 weeks. Other secondary outcomes are synovitis by clinical examination and ultrasound, measures of pain, function, stiffness, and health-related quality of life, and direct and indirect costs. Adverse events are recorded at each visit. The duration of the randomized controlled trial is 24 weeks, followed by an 80-week open-label observational phase to investigate the long-term efficacy and safety of repeated steroid injections and the occupational therapy intervention.
Conclusions: The results from this trial will have important clinical implications and influence future guidelines on OA management of the CMC-1 joint.
Clinical trial registration: EU-CT 2023-505254-17-00, NCT06084364.
Keywords: Clinical trial; Hand osteoarthritis; Osteoarthritis; Pain.
© 2024 The Authors.
Conflict of interest statement
AG reports consulting fees from Pfizer, TissueGene, Formation Bio, ICM, Coval, Novartis and Medipost, and stock/stock options in BICL and LLC, outside of the submitted work. KD is part funded by the National Institute for Health and Care Research (NIHR) Applied Health Research Collaboration (ARC) West Midlands (NIHR 200165) and the Birmingham Biomedical Research Centre (BRC). KD is also an NIHR Senior Investigator (ID NIHR 205031). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. HBH reports honorarium from Abbvie, Novartis, Lily and UCB, and advisory boards for Abbvie and Novartis, outside of the submitted work. HS reports patent issued and pending for Syrigma™ (syringe cover). IKH reports personal fees from Novartis, GSK and Grünenthal, and speaker honorarium from Abbvie, outside of the submitted work. TAS reports grants and personal fees from Roche, and personal fees from AbbVie, Roche, Sanofi, Takeda, and Novartis, outside the submitted work. AK, ATT, DTF, DS, EBF, ELE, IK, IPM, JKU, JS, KBH, KL, MG, MH, MHE, MIS, MO and TA report no conflicts of interest.
Figures

References
-
- Fjellstad C.M., Mathiessen A., Slatkowsky-Christensen B., Kvien T.K., Hammer H.B., Haugen I.K. Associations between ultrasound-detected synovitis, pain, and function in interphalangeal and thumb base osteoarthritis: data from the nor-hand cohort. Arthritis Care Res. 2020;72:1530–1535. - PubMed
-
- Kortekaas M.C., Kwok W.Y., Reijnierse M., Watt I., Huizinga T.W., Kloppenburg M. Pain in hand osteoarthritis is associated with inflammation: the value of ultrasound. Ann. Rheum. Dis. 2010;69:1367–1369. - PubMed
-
- Mathiessen A., Slatkowsky-Christensen B., Kvien T.K., Hammer H.B., Haugen I.K. Ultrasound-detected inflammation predicts radiographic progression in hand osteoarthritis after 5 years. Ann. Rheum. Dis. 2016;75:825–830. - PubMed
-
- Haugen I.K., Slatkowsky-Christensen B., Boyesen P., Sesseng S., van der Heijde D., Kvien T.K. MRI findings predict radiographic progression and development of erosions in hand osteoarthritis. Ann. Rheum. Dis. 2016;75:117–123. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

